Method for synthesizing metal phthalocyanine from phthalonitrile under catalysis of urea-choline chloride
A technology of choline chloride and phthalonitrile, which is applied in the direction of organic chemistry, can solve the problems of application limitations, poor solubility of phthalocyanine compounds, etc., and achieve the effect of ensuring the reaction temperature
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
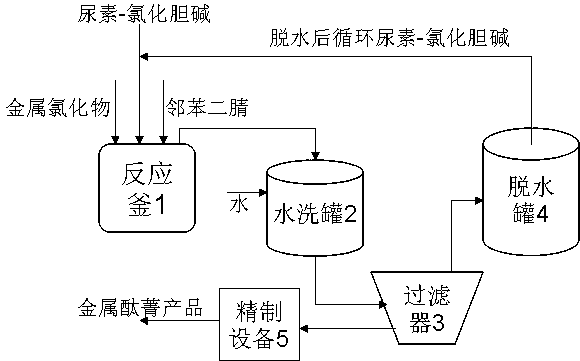
[0014] With 10 kilograms of 4-amino-phthalonitrile, urea and choline chloride mol ratio are 80 kilograms of urea-choline chloride deep eutectic solvent DES of 2.2:1, zinc chloride 2.6 kilograms, put into reactor 1 , heated to 120°C with steam, and reacted for 2 hours. After the material is diluted and washed with an appropriate amount of water in the washing tank 2, it is filtered in the filter 3, and the material containing urea-choline chloride deep eutectic solvent DES, etc. is dehydrated under negative pressure in the dehydration tank 4, and then sent back to the reaction kettle 1 is recycled; the filter cake is further purified in the refining equipment 5 to finally obtain 6.9 kilograms of cobalt phthalocyanine product.
Embodiment 2
[0016] 10 kilograms of 4-hydroxyl-phthalonitrile, urea and choline chloride mol ratio are 82 kilograms of urea-choline chloride deep eutectic solvent DES of 2:1, cobalt chloride 2.4 kilograms, drop into reactor 1 , heated to 115°C with steam, and reacted for 3 hours. After the material is diluted and washed with an appropriate amount of water in the washing tank 2, it is filtered in the filter 3, and the material containing urea-choline chloride deep eutectic solvent DES, etc. is dehydrated under negative pressure in the dehydration tank 4, and then sent back to the reaction kettle 1 is recycled; the filter cake is further purified in the refining equipment 5 to finally obtain 7.2 kilograms of cobalt phthalocyanine product.
Embodiment 3
[0018] 10 kilograms of 3-isoamyloxy-phthalonitrile, urea and choline chloride mol ratio are 84 kilograms of urea-choline chloride deep eutectic solvent DES of 1.8:1, copper chloride 2.6 kilograms, drop into In reactor 1, heat to 125° C. by steam, and react for 2 hours. After the material is diluted and washed with an appropriate amount of water in the washing tank 2, it is filtered in the filter 3, and the material containing urea-choline chloride deep eutectic solvent DES, etc. is dehydrated under negative pressure in the dehydration tank 4, and then sent back to the reaction kettle 1 is recycled; the filter cake is further purified in the refining equipment 5 to finally obtain 6.8 kilograms of copper phthalocyanine product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com