A kind of Rhodopseudomonas palustris atps2 protein and its preparation method and application
A swamp erythropseudomonas, protein technology, applied in the directions of botanical equipment and methods, biochemical equipment and methods, applications, etc., to achieve the effects of environmental friendliness, inhibition of pathogenicity, and short cultivation time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
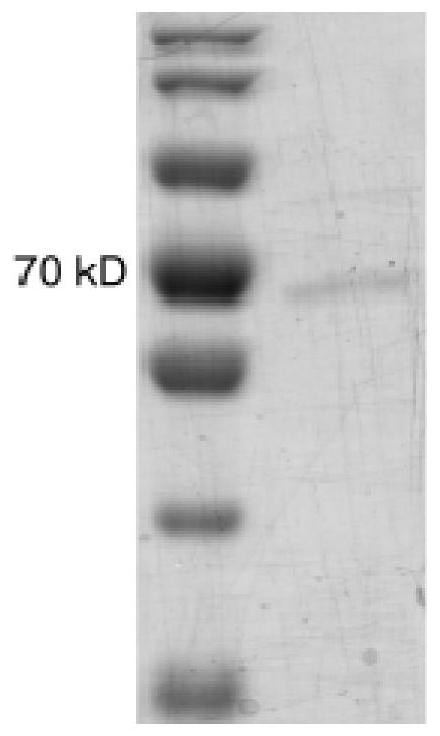
[0034] Preparation method of Rhodopseudomonas palustris Atps2 protein,
[0035] Include the following steps:
[0036] (1) Rhodopseudomonas palustris ATPS2 Prokaryotic expression of genes in E. coli
[0037] Construction vector: Rhodopseudomonas palustris ATPS2 The nucleotide sequence of the gene is connected to the PET32a plasmid containing the ampicillin resistance gene;
[0038] Escherichia coli transformation: the plasmid constructed in the previous step is introduced into the expression competent cell BL21, spread on the LB plate containing ampicillin resistance and cultivated; the medium of the LB plate containing ampicillin resistance, the formula For: yeast extract 5g / L, peptone 10g / L, NaCl 5g / L, ampicillin 100mg / L, agar 15g / L, pH=7.0;
[0039] Expansion of transformed strains and protein induction: transfer the single colony cultivated in the previous step into a 120mL Erlenmeyer flask and culture at 37°C until the stable phase; then inoculate the bacteria in the...
Embodiment 2
[0043] Application of Atps2 Protein from Rhodopseudomonas palustris in Inhibiting Magnaporthe grisea
[0044] (1) Application of Atps2 protein from Rhodopseudomonas palustris in inhibiting the formation of conidia of Magnaporthe grisea
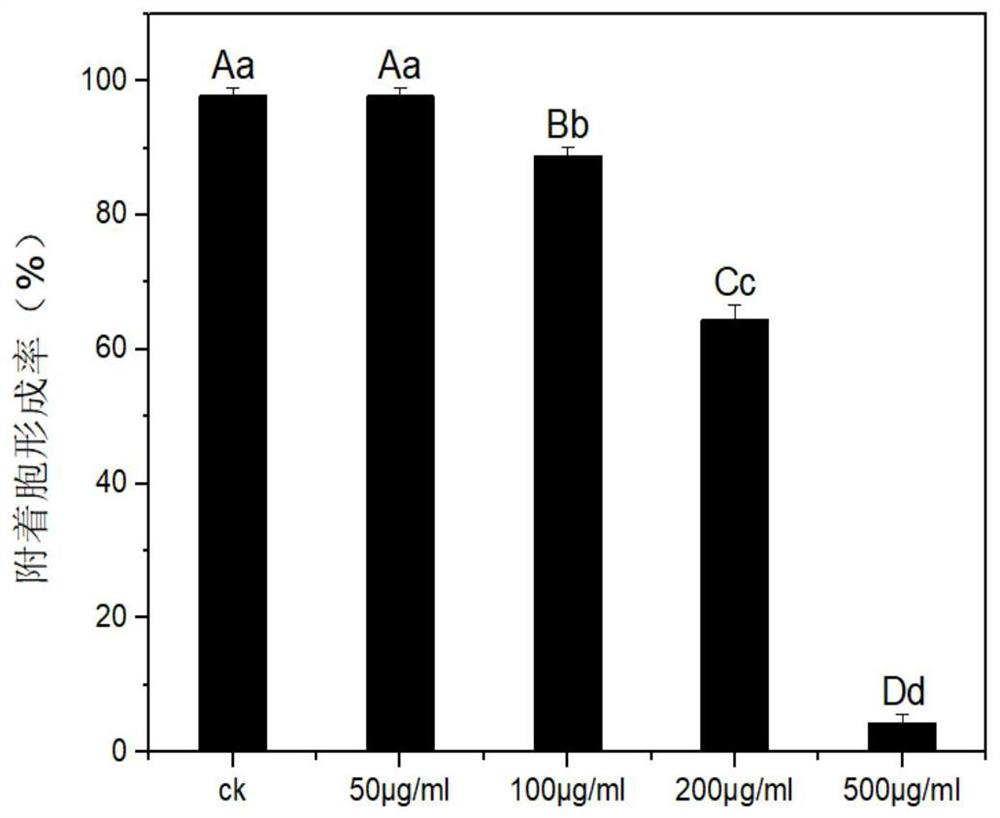
[0045] Select the Rhodopseudomonas palustris Atps2 protein prepared in Example 1 (final concentrations are 0 μg / ml, 50 μg / ml, 100 μg / ml, 200 μg / ml, 500 μg / ml), and add it to the conidia of Magnaporthe grisea Liquid (1 x 10 5 pcs / ml) for mixing, then 30 μl was dropped onto the hydrophobic membrane, and each group (corresponding to CK group, 50 μg / ml group, 100 μg / ml group, 200 μg / ml group, 500 μg / ml group) set 3 Repeat, observe microscopically after 8 hours and count the appressor formation rate, and evaluate the respective inhibitory effects;
[0046] Such as figure 2 As shown, the results show that the formation rate of appressoria after treatment with Rhodopseudomonas palustris Atps2 protein with a final concentration of 100 ug / mL is sig...
Embodiment 3
[0051] Referring to Example 2 "Application of Rhodopseudomonas palustris Atps2 Protein in Inhibiting the Formation of Pyricularia Oryzae Conidia Appresses", after prokaryotic expression, 3-hydroxyacyl dehydratase FabA with a concentration of 500 ug / mL was selected respectively , outer membrane protein assembly factor BamA and Atps2 protein were tested, and the appressor formation rate was counted. Among them, the 3-hydroxyacyl dehydratase FabA is derived from 3-hydroxyacyl-[acyl chain protein] dehydratase, the base pair is 525bp, and the protein molecular weight is 18.8kD; the outer membrane protein assembly factor BamA is derived from the outer membrane protein assembly factor, base The base pair is 2454 bp, and the protein molecular weight is 93.5kD.
[0052] Such as Figure 4 As shown, the formation rate of conidia of Magnaporthe grisea after treatment with Atps2 protein was less than 10%, which was significantly lower than that of CK control (** represents a very signific...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com