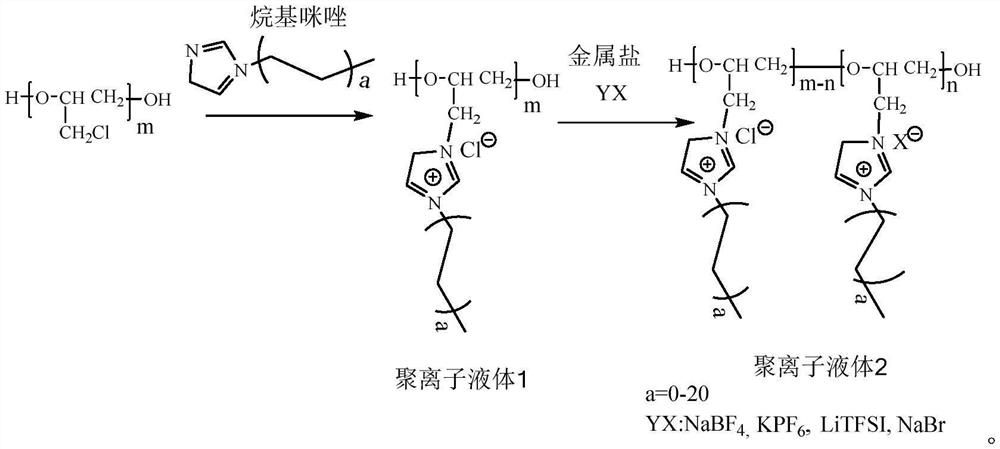
A kind of preparation method of imidazole polyionic liquid
An ionic liquid and imidazole technology, applied in the field of preparation of imidazole polyionic liquids, can solve the problems of limited types and quantities of imidazole polyionic liquids, and achieve the effect of enriching types and quantities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0015] Accurately weigh (10 g, 1 mmol) hydroxy-terminated polyepichlorohydrin, add it to a 250 mL round-bottomed flask, pass argon into the round-bottomed flask for 10 min, and add methylimidazole (0.2 g, 2.8 mmol) , seal the round-bottomed flask and place it in an oil bath at 80°C for 12 hours of reaction. After the reaction is stopped, settle it in ether solution, collect the precipitate by centrifugation, wash it with ether for many times, and dissolve it in ethanol. Continue to use Diethyl ether precipitation treatment, centrifugal washing for several times to completely wash small molecular impurities, and drying in a vacuum drying oven at 60 °C for 24 hours to obtain side chains containing Cl - The imidazole polyionic liquids 1.
Embodiment 2
[0017] Accurately weigh the side chain containing Cl prepared in 1 - The imidazole-based polyionic liquid 1 (1.9 g, 10 mmol) was added to a 250 mL round-bottomed flask, 30 mL of distilled water was added to dissolve it to form a polyionic liquid 1 solution, and the bistrifluoromethanesulfonimide lithium LiNTf was weighed. 2 (1.43g, 5mmol) was dissolved in 20mL of distilled water, then added dropwise to the polyionic liquid 1 solution, stirred at room temperature for 4h, stopped stirring, placed in an oil bath at 80°C for 1h, and removed the upper layer of water Then, add 60 mL of distilled water to the round-bottomed flask and stir for 20 min. After standing for 30 min, remove the upper water phase. Use silver nitrate solution to detect whether there are chloride ions in the upper water phase. If so, continue to repeat the above experimental steps until the upper water is Until there is no chloride ion in the phase, rotary-evaporated to remove part of the aqueous solution, and...
Embodiment 3
[0019] Accurately weigh the side chain containing Cl prepared in 1 - The imidazole-based polyionic liquid 1 (1.9 g, 10 mmol) was added to a 250 mL round-bottomed flask, 30 mL of distilled water was added to dissolve it to form a polyionic liquid 1 solution, and the bistrifluoromethanesulfonimide lithium LiNTf was weighed. 2 (0.71 g, 2.5 mmol) was dissolved in 20 mL of distilled water, and then added dropwise to the solution of polyionic liquid 1. During the dropwise addition, a light yellow oily liquid appeared, stirred at room temperature for 4 h, stopped stirring, and placed in a 80°C Let stand in an oil bath for 1 hour to remove the upper aqueous phase, then add 60 mL of distilled water to the round-bottomed flask and stir for 20 min. After standing for 30 min, remove the upper aqueous phase and use silver nitrate solution to detect whether there is still chloride ion in the upper aqueous phase. If there is, continue to repeat the above experimental steps until there is no ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com