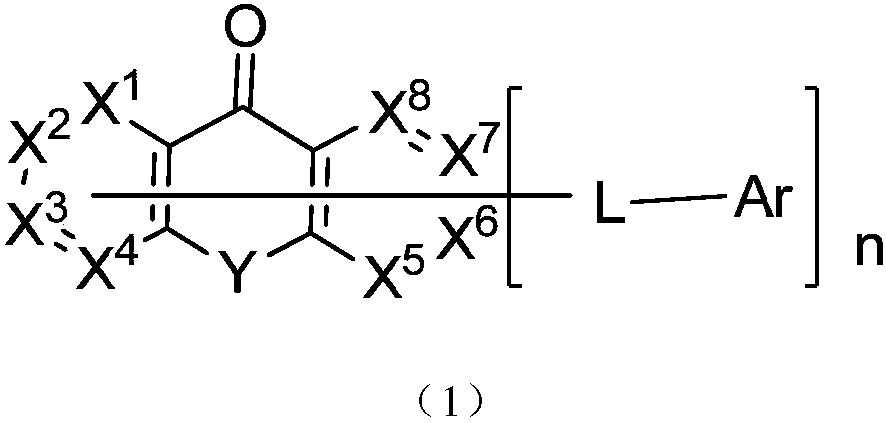
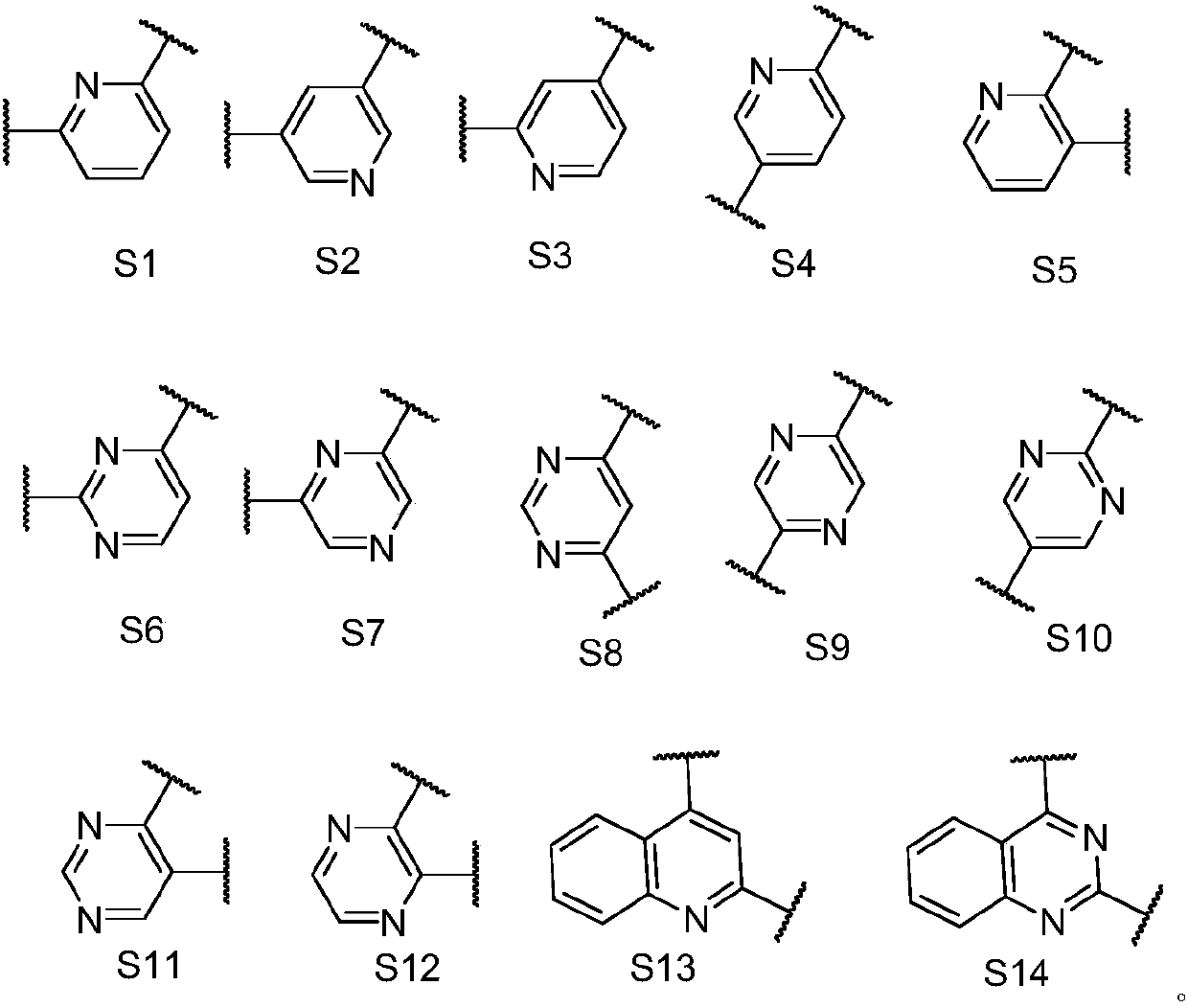
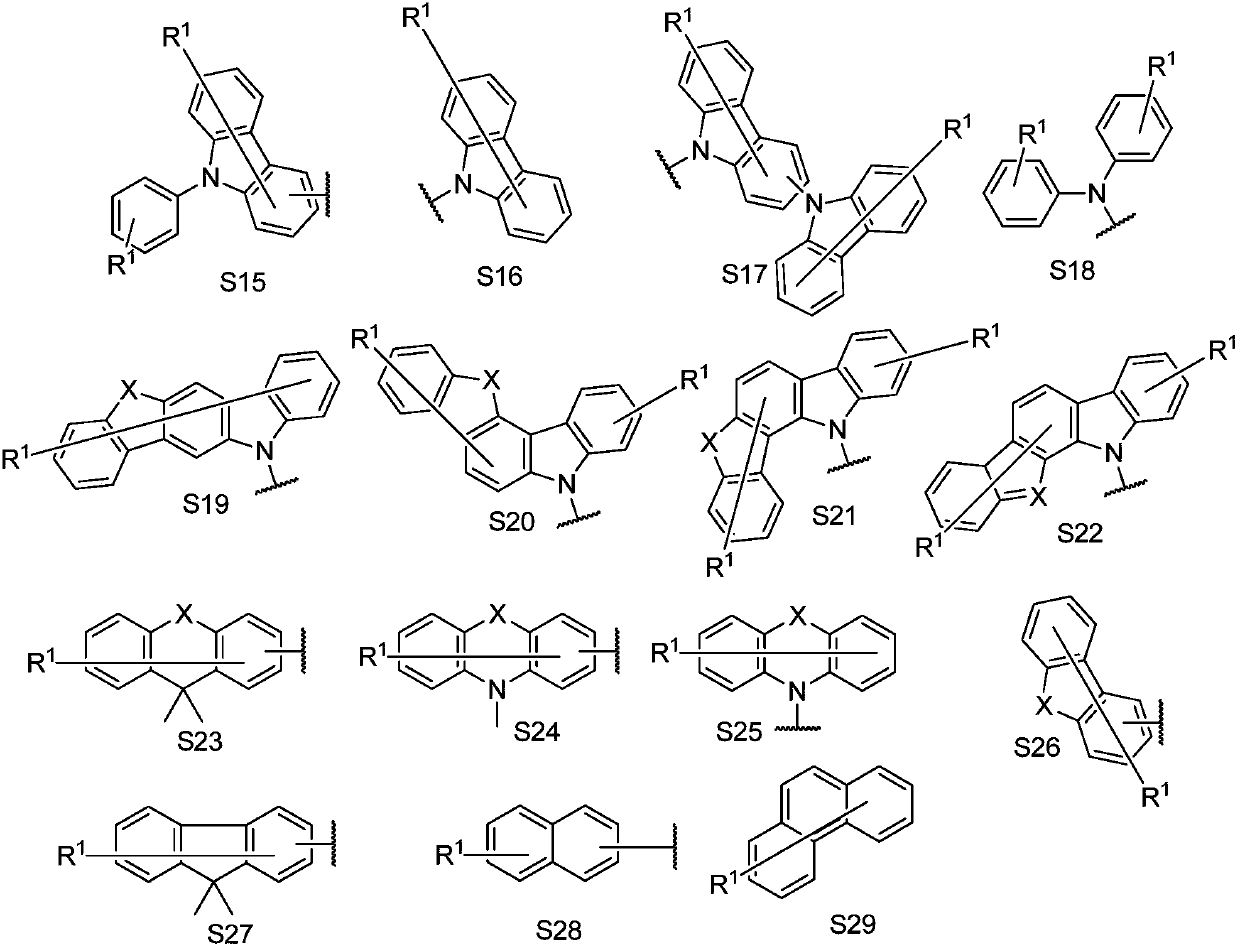
Anthraquinone general formula compound and application thereof
A compound of the general formula and a selected technology, applied in the field of organic electroluminescent devices, can solve problems such as efficiency roll-off, reduce the efficiency roll-off, improve rigidity, and realize the effect of narrow-spectrum luminescence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] Preparation of intermediate M1:
[0050] In a 500mL three-necked flask, add 2-fluoro-4-bromobenzonitrile (10g, 50mmol), phenol (4.94g, 52.5mmol) and K 2 CO 3 (13.8g, 100mmol) and DMF (120mL), heated at 140°C for 4 hours under the protection of nitrogen. TLC monitored the reaction to completion. The reaction solution was cooled to room temperature, diluted with toluene (100mL), washed with water (50mL*2), separated to remove the water phase, and the organic phase was dried with anhydrous sodium sulfate and concentrated to a small volume. White solid, filtered to obtain intermediate M1 13.2g.
[0051] Preparation of Intermediate M2:
[0052] Intermediate M1 (63g, 229mmol) was dissolved in EtOH (500mL), NaOH (27g, 689mmol) was added, heated to 100°C under nitrogen protection, and refluxed for 5h. TLC monitored until the reaction was complete. After cooling down, filter to obtain a solid. Adjust the system to PH=1 with hydrochloric acid (1N), extract the aqueous phas...
Embodiment approach
[0100] The organic light emitting diode includes a first electrode and a second electrode on the substrate, and an organic material between the electrodes, and a hole transport layer, a light-emitting layer, and an electron transport layer are included between the first electrode and the second electrode.
[0101] The substrate is the substrate used in organic light emitting displays, such as glass, polymer materials, and glass and polymer materials with TFT components.
[0102] The anode material can be indium tin oxide (ITO), indium zinc oxide (IZO), tin dioxide (SnO 2 ), zinc oxide (ZnO) and other transparent conductive materials, also metal materials such as silver and its alloys, aluminum and its alloys, organic conductive materials such as PEDOT, and multilayer structures of the above materials.
[0103] The device can also include a hole injection layer positioned between the hole transport layer and the anode, including but not limited to one or more combinations of HI...
Embodiment 2
[0132] ITO(150nm) / HI-2(10nm) / HT-2(40nm) / HT-28(20nm) / TDH14:20%C2(30nm) / ET-34(20nm) / LiF(0.5nm) / Al( 150nm)
[0133] Wherein 5% means that the weight ratio of C1 relative to TDH14 is 20%, and the following examples are also expressed in this manner.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com