A tumor microenvironment h 2 o 2 Responsive cross-linked near-infrared molecular probes and their applications
A tumor microenvironment and molecular probe technology, which is applied in the field of molecular probes and near-infrared fluorescence imaging, can solve the problems of short residence time, influence of tumor imaging effect, rapid excretion of biological tissues, etc., to prolong residence time, Effect of Good Fluorescence Imaging Performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
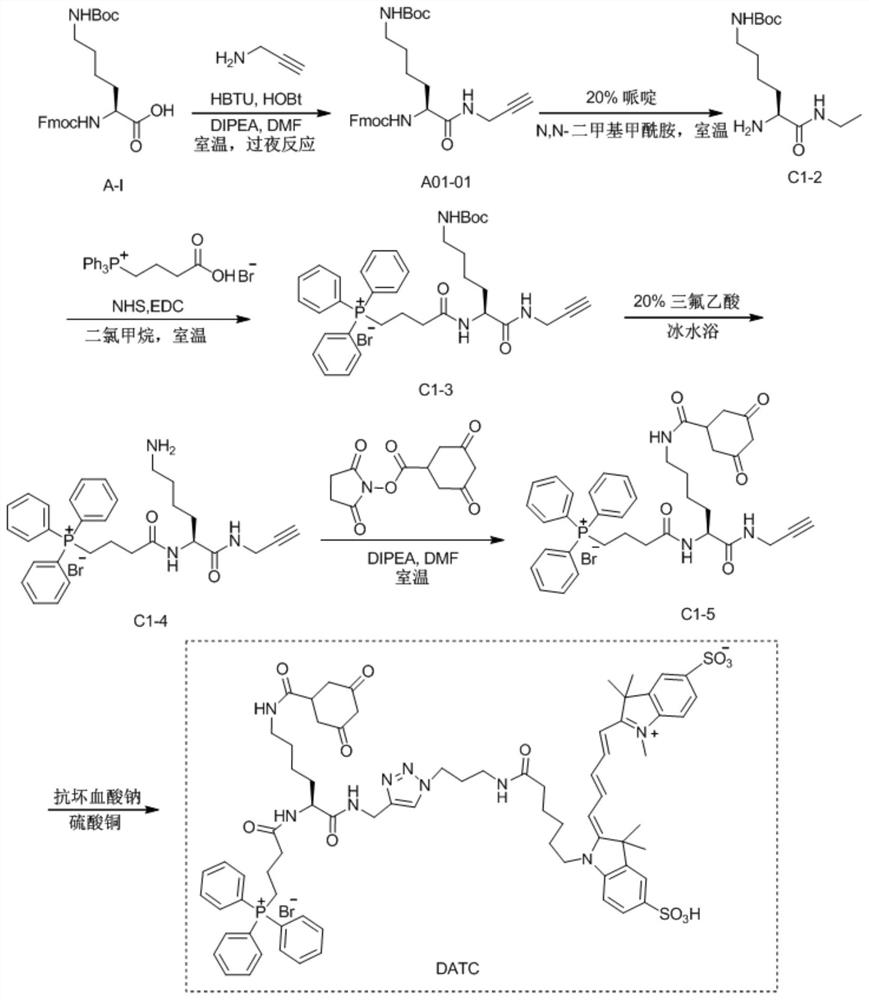
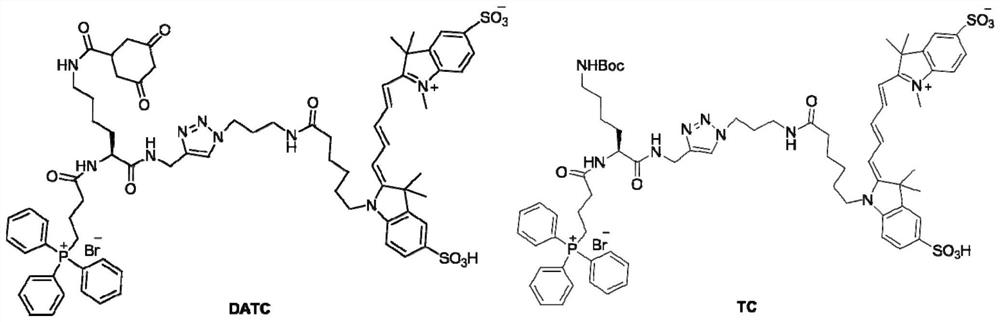
[0065] Example 1: H 2 o 2 Synthesis and characterization of responsive near-infrared molecular probe DATC and control probe TC
[0066] (1) Add N-tert-butoxycarbonyl-N'-fluorenylmethoxycarbonyl-D-lysine (2.34g, 5mmol), N,N-dimethylformamide (30mL), Benzotriazole-1-tetramethylhexafluorophosphate (2.28g, 6mmol), 1-hydroxybenzotriazole (0.81g, 6mmol) and diisopropylethylamine (1.02mL, 6mmol) were stirred in an ice-water bath 15min. Subsequently, propargylamine (330 μL, 6 mmol) was added into the reaction flask, and the reaction was continued at room temperature for 15 hours under stirring. After the reaction was completed, the solvent was removed by rotary evaporation, and 150 mL of ethyl acetate was added to redissolve the intermediate product. Subsequently, the organic phase was washed once with 30mL deionized water, saturated sodium bicarbonate and sodium chloride aqueous solution, dried with anhydrous sodium chloride, and spin-dried to obtain white powdery intermediate A0...
Embodiment 2
[0076] Example 2: H 2 o 2 Cross-linking of Responsive Near Infrared Molecular Probe DATC under the Action of Hydrogen Peroxide
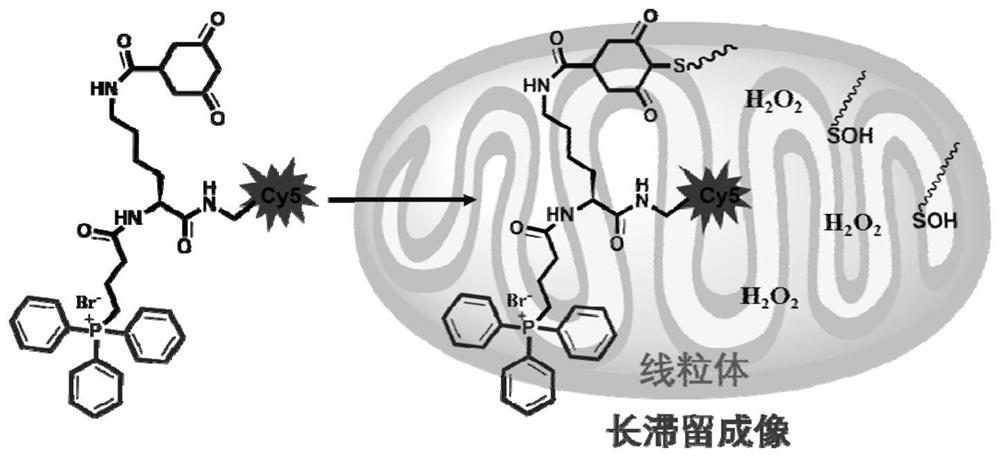
[0077] 4T1 cells were first treated with 100 μM hydrogen peroxide for 15 min, and then the H 2 o 2 The responsive near-infrared molecular probe DATC was diluted into the cell culture medium, and then added to the 4T1 cell culture dish for incubation (the probe concentration was 5 μM). The sulfhydryl group on the 4T1 intracellular protein treated with hydrogen oxide will be oxidized to sulfenic acid, and when the probe enters the tumor cell, the group 3,5-dioxocyclohexanecarboxylic acid will combine with Acid cross-linking forms a C-S covalent bond, which firmly connects the probe molecule with the macromolecular protein in the tumor cell, thereby prolonging the residence time of the molecular probe in the tumor cell and improving the imaging effect of the probe molecule. For technical effects, see Figure 8 .
Embodiment 3
[0078] Example 3: H 2 o 2 Purity Characterization and Mass Spectrometry Characterization of Responsive Near Infrared Molecular Probe DATC in High Performance Liquid Chromatography
[0079] The H prepared in Example 1 2 o 2 The responsive near-infrared molecular probe was diluted with solvent methanol to a concentration of 5 μM, its molecular weight was determined by mass spectrometry, and its purity was analyzed by high performance liquid chromatography.
[0080] Such as Figure 4 As shown in a, the sample was analyzed by Agilent 1260 high performance liquid chromatography, the retention time of the probe DATC was 5.260 minutes, and the sub-area was further integrated to calculate the concentration of the probe in the sample as high as 98%. Figure 4 b shows the theoretical m / z of the probe DATC: 1378.64, and the m / z ([M+ TFA+2Cl]-) in the mass spectrum actually obtained: 1562.0, the two are consistent, which is the desired compound.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com