Mutants of formate dehydrogenase with improved substrate affinity and coenzyme affinity
A formate dehydrogenase and mutant technology, applied in the field of bioengineering, can solve problems such as unsuitable for practical application, high concentration of formate substrate and coenzyme, increased cost of coenzyme regeneration and reaction liquid treatment cost, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Example 1 Site-directed mutation of BstFDH
[0071] Through Uniprot, NCBI BLAST and spatial structure modeling, the three-dimensional structure of BstFDH with the amino acid sequence shown in the sequence table SEQ ID No.2 was obtained, and the amino acid residues around the binding site of the coenzyme and the substrate were mutated. Site-directed saturation mutation was performed using the recombinant plasmid of the female parent BstFDH as a PCR template. The PCR system was: 10 μl of 2×PrimeStar, 1 μl of upstream primer and downstream primer (10ng / μl), 1 μl of template plasmid (50ng / μl), 1 μl of DMSO and ddH 2 O 6 μl. The PCR amplification program was as follows: 30 cycles of denaturation at 98°C for 5 minutes followed by denaturation at 98°C for 30 seconds, annealing at 55°C for 30 seconds, extension at 72°C for 7 minutes, and finally extension at 72°C for 10 minutes. The PCR amplified product was digested with DpnI for 2 hours, the digested product was transformed ...
Embodiment 2B
[0073] Combination mutation of embodiment 2BstFDH
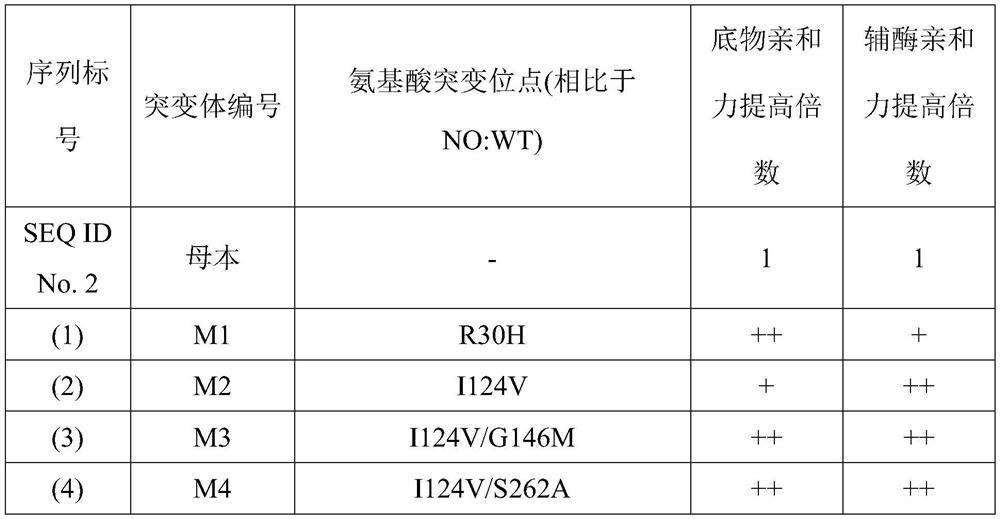
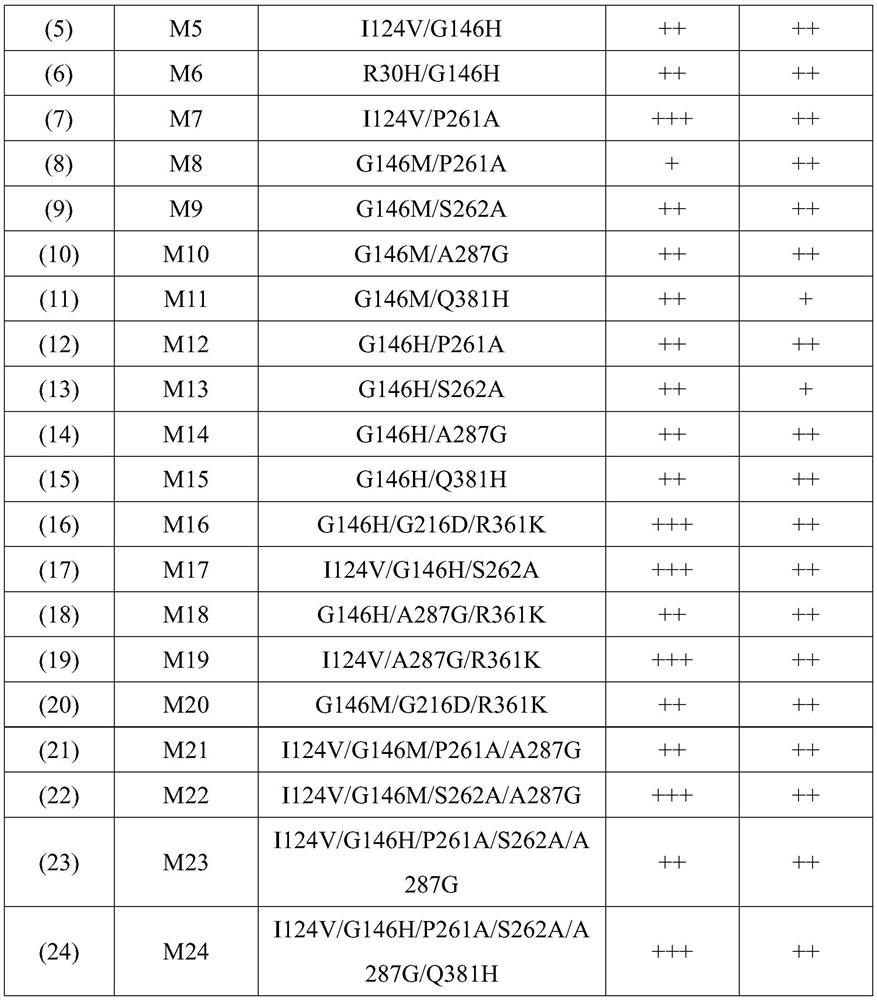
[0074] On the basis of the mutation in Example 1, some mutation points were combined to obtain mutants with significantly improved affinity for coenzyme and substrate. in FIG. 1. In the list in Table 1, the sequence numbers refer to the corresponding series of sequences at the back of Table 1; in the mutant affinity improvement multiple, a plus sign "+" indicates that the ratio of the mutant protein is shown in SEQ ID No.2 in the sequence table The affinity of the protein composed of the amino acid sequence to the substrate or coenzyme is increased by 0.1-1 times; two plus signs "++" indicate that the mutant protein is more sensitive to the substrate than the protein composed of the amino acid sequence shown in SEQ ID No.2 in the sequence table The affinity of the substance or coenzyme has been improved by 1-4 times; three plus signs "+++" indicate that the affinity of the mutant protein to the substrate or coenzyme is impro...
Embodiment 2
[0103] Example 2 Expression and catalytic efficiency of recombinant BstFDH k cat / K m Determination of
[0104] Inoculate the recombinant Escherichia coli E. coli BL21(DE3) / pET22b-BstFDH into 100mL LB medium containing 50μg / ml ampicillin, culture on a shaker at 37°C for 12 hours, and then inoculate at 1% (v / v) The amount was added to a 500ml Erlenmeyer flask equipped with 100ml LB medium (containing 50μg / ml ampicillin), placed in a shaker at 37°C and 180rpm for shaking culture, when the OD of the culture solution 600 When it reached 0.6, IPTG with a final concentration of 0.2mmol / L was added as an inducer and induced at 16°C for 24h. The culture solution was centrifuged at 8000×g for 10 min, the cells were collected, and washed twice with saline to obtain resting cells. Suspend the cells obtained in 100ml of culture medium in 10ml of potassium phosphate buffer (100mM, pH 7.0), and perform the following ultrasonic disruption in an ice-water bath: 400W power, working for 4s, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com