1,3-biscarbazolyl benzene type phosphorescent host material, and synthesis method and application thereof
A technology based on dicarbazole benzenes and phosphorescent main body, applied in the application field of optoelectronic materials, which can solve the problems of hindering widespread use, low material stability, high efficiency roll-off, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
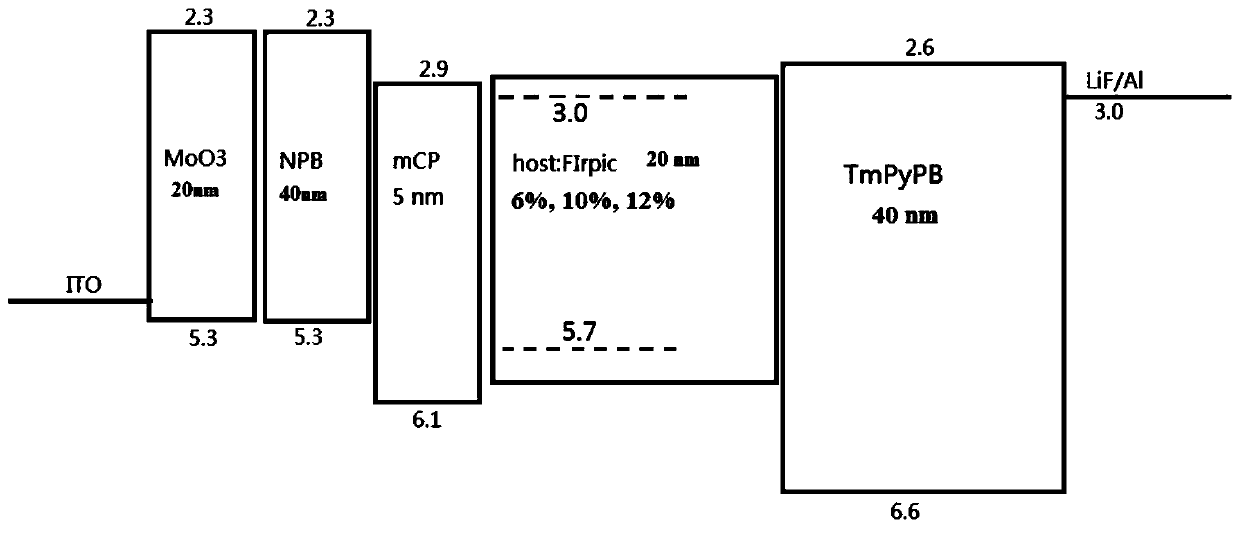
Examples
preparation example Construction
[0060] The synthesis method of the 1,3-dicarbazole benzene phosphorescent host material disclosed by the present invention comprises the following steps:
[0061] Step 1: Dissolve m-dibromobenzene and carbazole in DMPU (1,3-dimethylpropylene urea), heat to 180°C for reflux for 48 hours, and use Ullmann reaction to obtain mCP (1,3-dicarboxylate azoles);
[0062] Step 2: Dissolving NBS (N,N'-dimethylbenzamide) and mCP in DMF and reacting at room temperature for 6 hours to obtain 3-bromo-1,3-dicarbazolylbenzene;
[0063] Step 3: Dissolving 3-bromo-1,3-dicarbazolylbenzene and diboronic acid ester in THF (tetrahydrofuran) and refluxing for 24 hours to form 3-boronic acid ester-1,3-dicarbazolylbenzene;
[0064] Step 4: using the Suzuki reaction to obtain 3-phenyl-1,3-dicarbazolylbenzene substituted by o-, m-, and p-bromine;
[0065] Step 5: Ullmann reaction, Suzuki reaction or diphenylphosphine-oxygen reaction are used to form the host luminescent material substituted by ortho-, m...
Embodiment 1
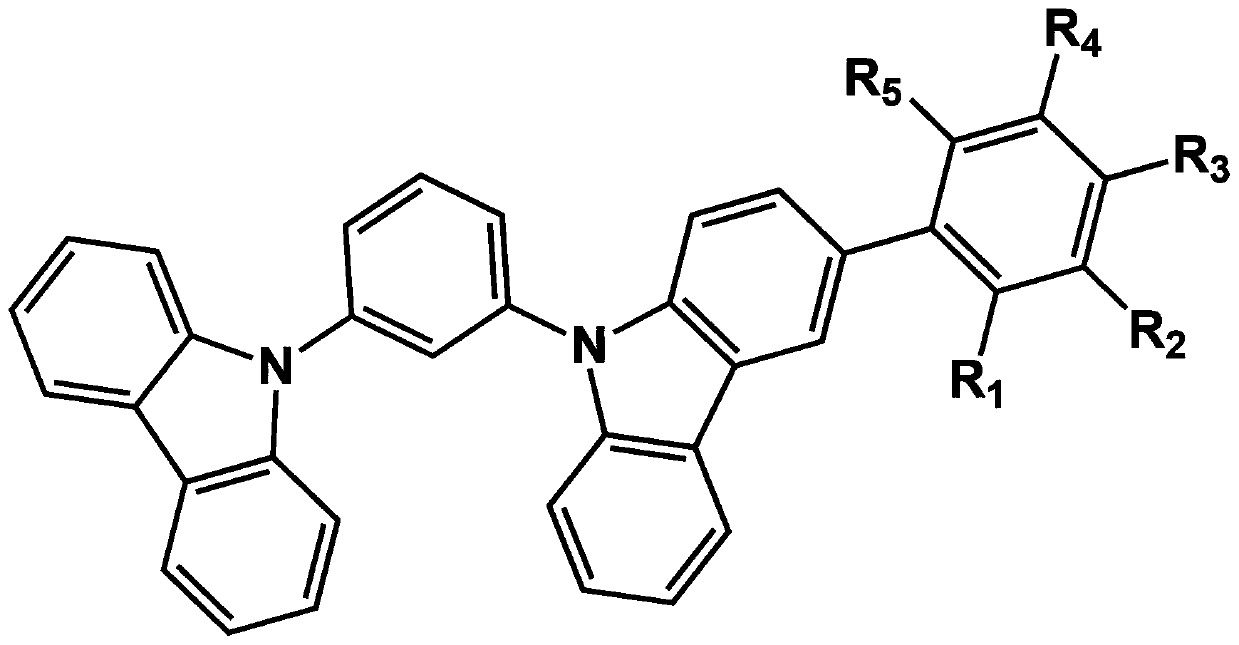
[0073] Embodiment 1: when R in formula I 1 For diphenylphosphoryloxy, R 2 , R 3 , R 4 and R 5 When it is a hydrogen group, it is named 3-(2-diphenylphosphoryloxy)phenyl-1,3-dicarbazolylbenzene (2-(9-(3-(9H-carbazol-9-yl)phenyl)- The structural formulas of 9H-carbazol-3-yl)phenyl)diphen-ylp hosphine oxide (mCPoPO) are as follows:
[0074]
[0075] The mCPoPO of the present invention can be synthesized by the following method.
[0076] Step a: Dissolve 1 mol of m-dibromobenzene and 2.5 mol of carbazole in 250 mL of DMPU (1,3-dimethylpropylene urea), heat to 180°C for reflux for 48 hours, and use the Ullmann reaction to obtain 0.94 mol of mCP ( 1,3-dicarbazole benzene);
[0077] Step b: Dissolve 0.9mol NBS (N,N'-dimethylbenzamide) and 0.92mCP in DMF and react at room temperature for 6h to obtain 0.89mol 3-bromo-1,3-dicarbazolylbenzene ;
[0078] Step c: Dissolve 0.85mol 3-bromo-1,3-dicarbazolylbenzene and 1.0mol diboronic ester in 1LTHF (tetrahydrofuran) and reflux for...
Embodiment 2
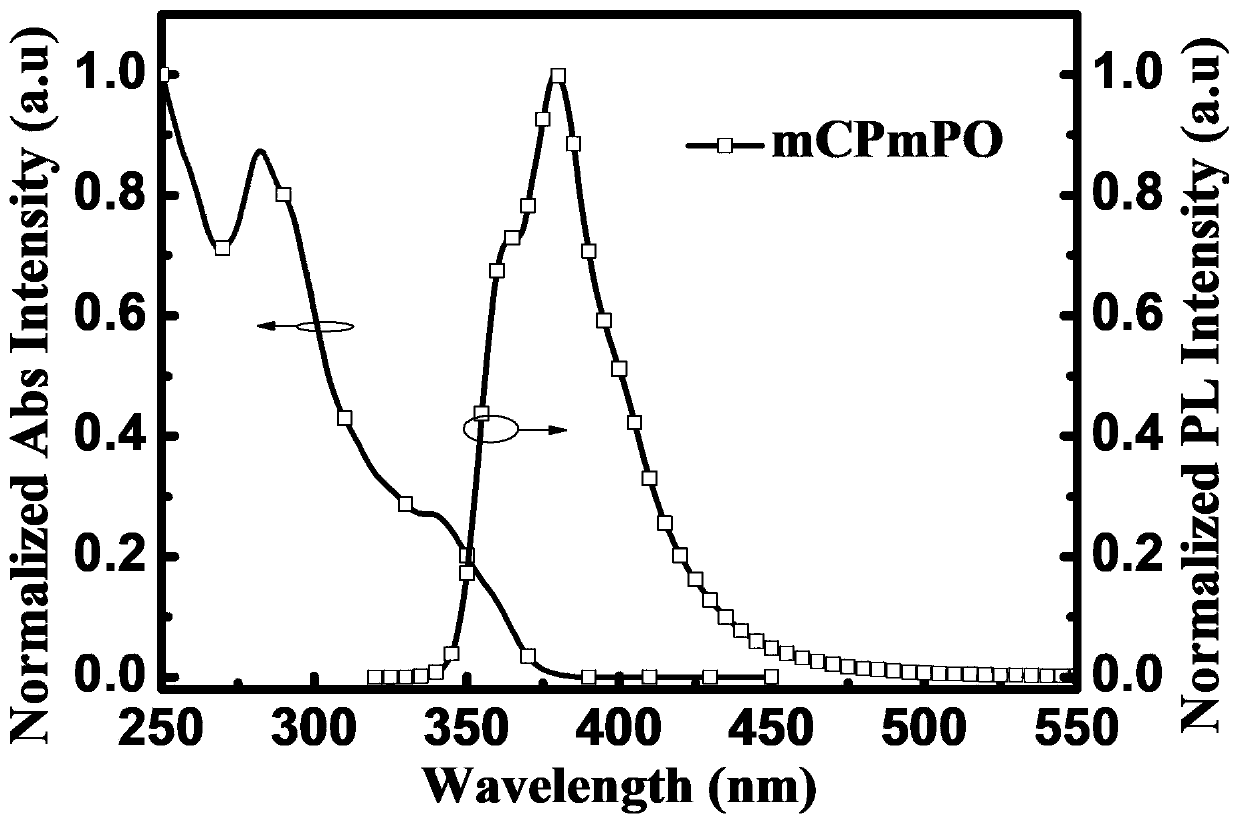
[0082] Embodiment 2: when R in formula I 2 For diphenylphosphoryloxy, R 1 , R 3 , R 4 and R 5 When it is a hydrogen group, it is named 3-(3-diphenylphosphoryloxy)phenyl-1,3-dicarbazolylbenzene (3-(9-(3-(9H-carbazol-9-yl)phenyl) -9H-carbazol-3-yl)phenyl)diphenylph osphine oxide (mCPmPO), its structural formula is as follows:
[0083]
[0084] The mCPmPO of the present invention can be synthesized by the following method.
[0085] Step a: Dissolve 1 mol of dibromobenzene and 2.5 mol of carbazole in 250 mL of DMPU (1,3-dimethylpropylene urea), heat to 180°C for reflux for 48 hours, and use the Ullmann reaction to obtain 0.94 mol of mCP ( 1,3-dicarbazole benzene);
[0086] Step b: Dissolve 0.9mol NBS (N,N'-dimethylbenzamide) and 0.92mCP in DMF and react at room temperature for 6h to obtain 0.89mol 3-bromo-1,3-dicarbazolylbenzene ;
[0087] Step c: Dissolve 0.85mol 3-bromo-1,3-dicarbazolylbenzene and 1.0mol diboronic ester in 1LTHF (tetrahydrofuran) and reflux for 24h to f...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Glass transition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com