Metal-organic framework material with hydrogen bond catalytic activity, and preparation method and application thereof
A catalytic activity, organic framework technology, applied in the preparation of organic compounds, catalytic reactions, organic compounds/hydrides/coordination complex catalysts, etc. , inactivation and other problems, to achieve the effect of low price, good size selectivity and reproducibility, and low cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
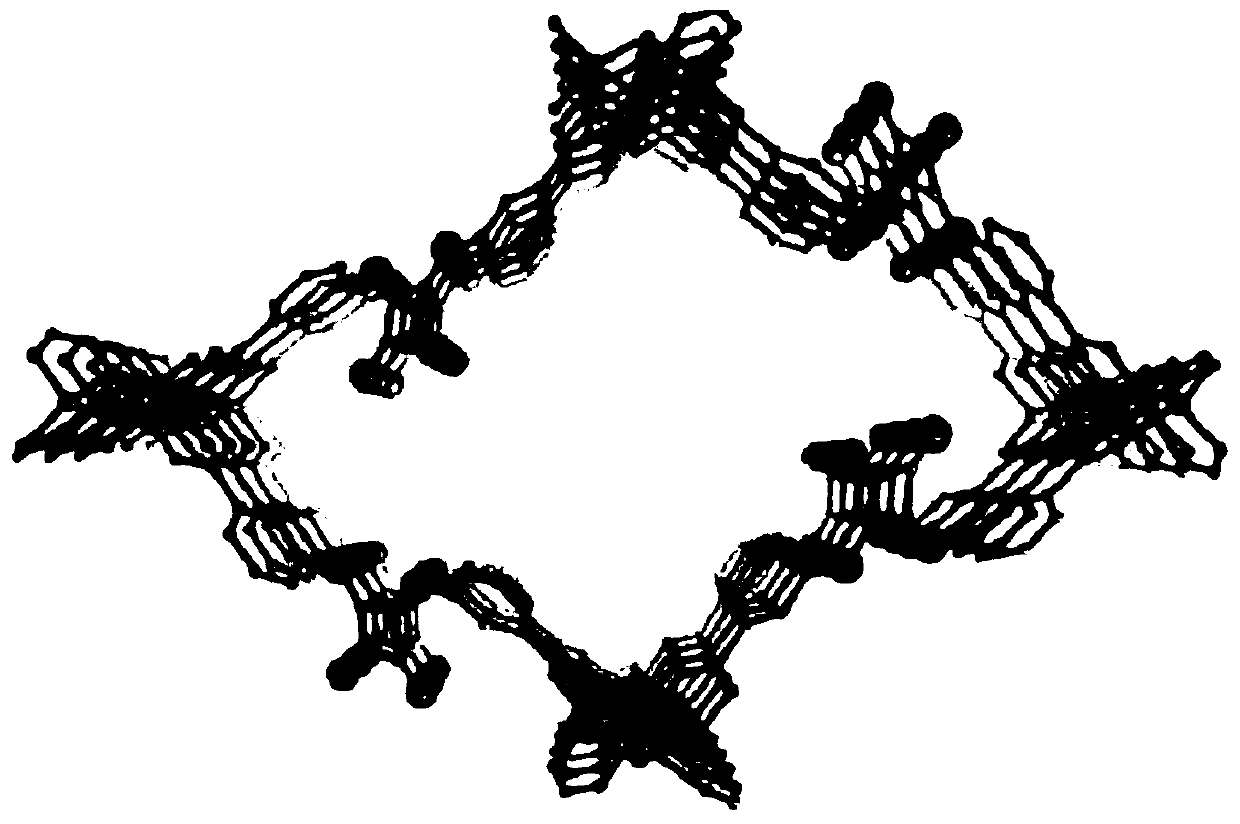
[0040] Weigh 0.1mmol 3,3'-((3,4-dioxocyclobut-1-ene-1,2-diyl)bis(imino))dibenzoic acid (H 2 dbda) and 0.4mmol Zn(NO 3 ) 2 ·6H 2 0, placed in a 10mL glass sample bottle, add 5mL of ethanol and DMF (volume ratio is 1:4) mixed solvent, then the glass sample bottle is placed in the hydrothermal synthesis reaction kettle, and then the reaction kettle is placed on the electric heating drum In an air drying oven, keep the temperature at 80°C and react for 24 hours. After the reaction is over, cool down to obtain a light yellow blocky crystal, then filter, wash with ethanol, and dry at 100°C for 9 hours to prepare the metal-organic framework material Zn-DBDA. The yield is: 85%. Its crystal structure is as figure 1 As shown, it can be seen from the figure that there are two groups of aromatic amine groups with different orientations in each independent channel, which provide potential hydrogen bonding sites for the activation of substrates through hydrogen bonding.
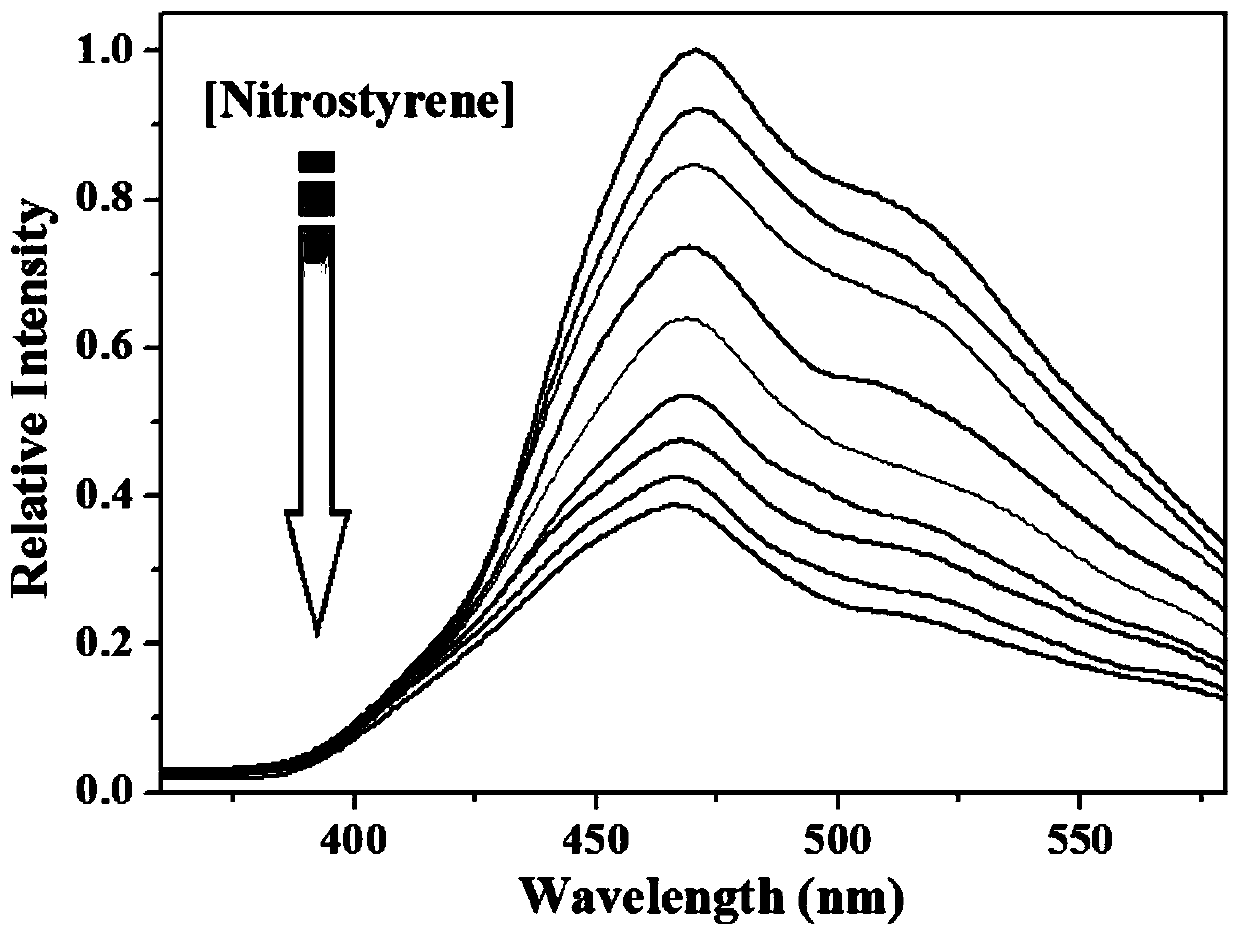
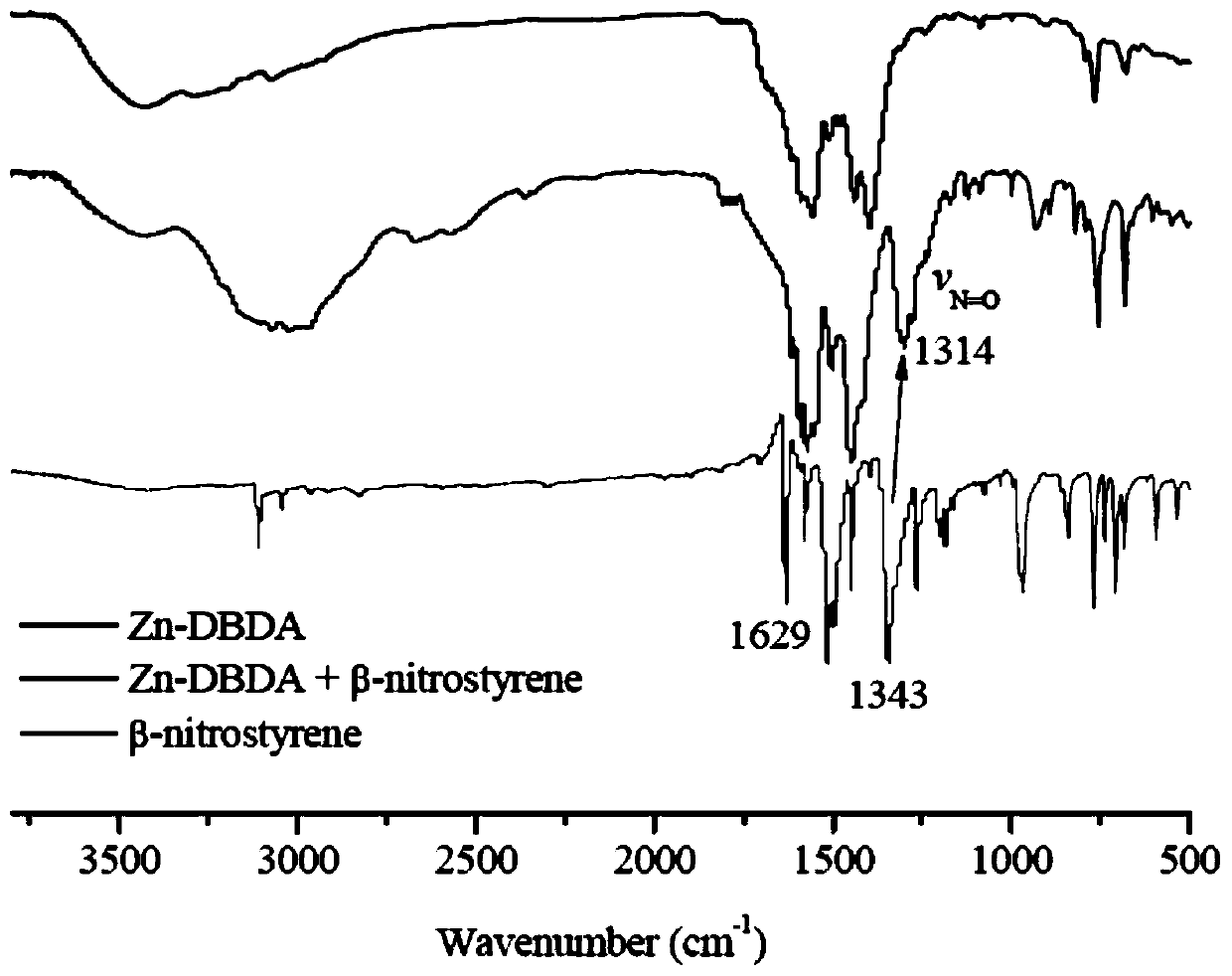
[0041] Cataly...
Embodiment 2
[0054] Weigh 0.1mmol 3,3'-((3,4-dioxocyclobut-1-ene-1,2-diyl)bis(imino))dibenzoic acid (H 2 dbda) and 0.38mmol Zn(NO 3 ) 2 ·6H 2 0, placed in a 10mL glass sample bottle, add 5mL of ethanol and DMF (volume ratio is 1:4) mixed solvent, then the glass sample bottle is placed in the hydrothermal synthesis reaction kettle, and then the reaction kettle is placed on the electric heating drum In an air drying oven, keep a constant temperature of 75°C and react for 22 hours. After the reaction is completed, cool down to obtain a light yellow blocky crystal, then filter, wash with ethanol, and dry at 95°C for 10 hours to prepare the metal-organic framework material Zn-DBDA. The yield is: 82%. Its crystal structure and catalytic performance are the same as in Example 1.
Embodiment 3
[0056] Weigh 0.1mmol 3,3'-((3,4-dioxocyclobut-1-ene-1,2-diyl)bis(imino))dibenzoic acid (H 2 dbda) and 0.42mmol Zn(NO 3 ) 2 ·6H 2 0, placed in the glass sample vial of 10mL, add the mixed solvent of 5.2mL ethanol and DMF (volume ratio is 1:4.2), then the glass sample vial is placed in the hydrothermal synthesis reactor, then the reactor is placed on the electric heater In a blast drying oven, keep a constant temperature of 80°C and react for 24 hours. After the reaction is over, cool down to obtain a light yellow blocky crystal, then filter, wash with ethanol, and dry at 100°C for 8 hours to obtain the metal-organic framework material Zn–DBDA. The yield : 84%. Its crystal structure and catalytic performance are the same as in Example 1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com