Carbazole Derivatives and Their Applications in Electroluminescent Devices
An electroluminescent device, a carbazole-based technology, applied in the field of organic electroluminescent materials, can solve the problems of efficiency stability not perfectly meeting the requirements of industrialization, unbalanced carrier transport matching ability, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0054]
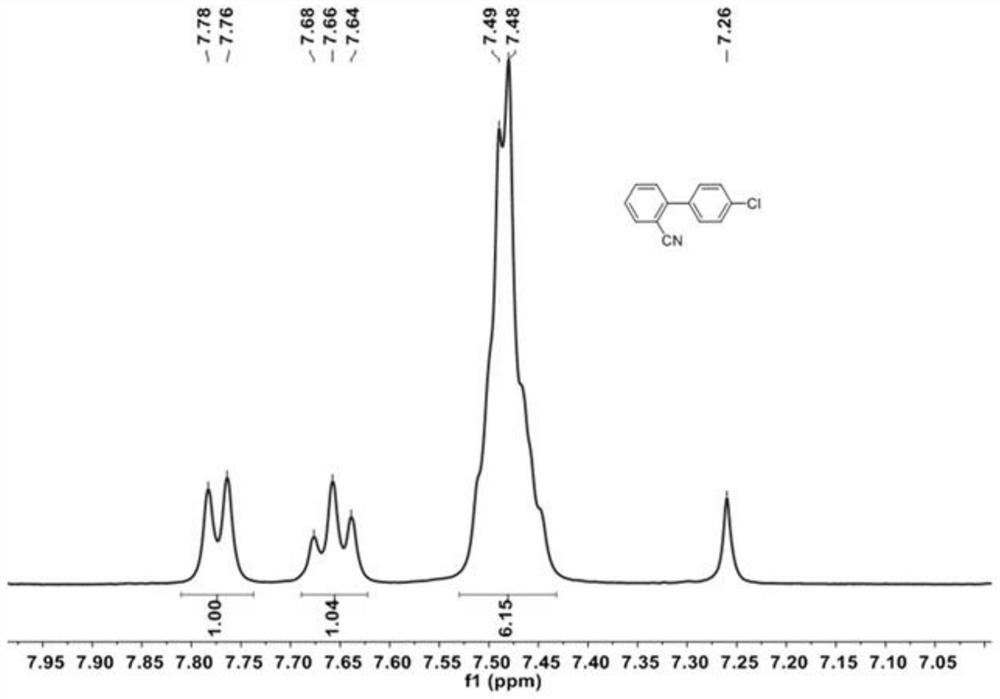
[0055] 2-iodobenzonitrile (5g, 21.8mmol), (4-chlorophenyl) boronic acid (4.08g, 26.16mmol), K 2 CO 3 (12.03g, 87.2mmol) and tetrakis (triphenylphosphine) palladium Pd (PPh 3 ) 4 (254mg, 0.22mmol) was dissolved in a mixture of 1,4-dioxane and water (200ml, 1,4-dioxane / water=10 / 1, v / v). The resulting solution was heated at 90 °C overnight. After cooling to room temperature, the solution was mixed with 200 mL of water and the desired product was extracted with dichloromethane. The organic layers were collected and evaporated under reduced pressure. The resulting crude product was purified by column chromatography using petroleum ether / dichloromethane (PE / DCM, 3 / 1, V / V) as eluent to give 4'-chloro-[1,1'-biphenyl]- 2-Carbonitrile as a white solid (4.57 g, 98%), which 1 H-NMR test results such as figure 1 shown.
Embodiment 2
[0057]
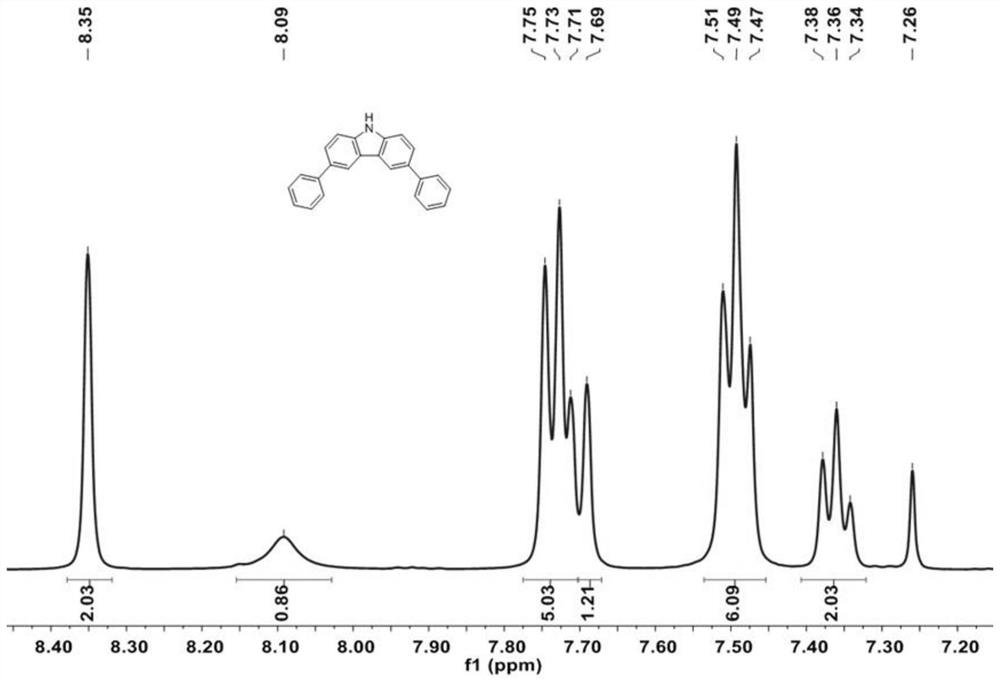
[0058] 3,6-dibromo-9H-carbazole (2g, 6.15mmol), phenylboronic acid (1.80g, 14.76mmol), K 2 CO 3 (3.4g, 24.6mmol) and tetrakis(triphenylphosphine)palladium Pd(PPh 3 ) 4 (208 mg, 0.18 mmol) was dissolved in a mixture of 1,4-dioxane and water (100 mL, 1,4-dioxane / water=10 / 1, v / v). The resulting solution was heated at 90 °C overnight. After cooling to room temperature, the solution was mixed with 200 mL of water and the desired product was extracted with dichloromethane. The organic layers were collected and evaporated under reduced pressure. The resulting crude product was purified by column chromatography using petroleum ether / dichloromethane (PE / DCM, 2 / 1, V / V) as eluent to give 3,6-diphenyl-9H-carbazole as a white solid (1.69g, 86%), which 1 H-NMR test results such as figure 2 shown.
Embodiment 3
[0060]
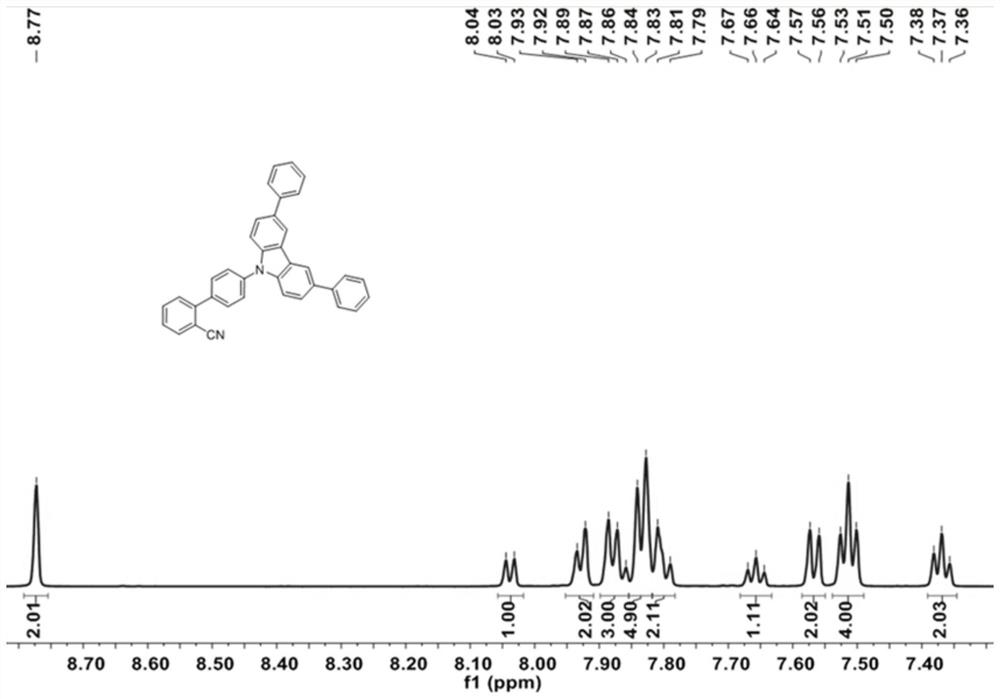
[0061] 3,6-diphenyl-9H-carbazole (1g, 3.13mmol), 4'-chloro-[1,1'-biphenyl]-2-carbonitrile (805mg, 3.76mmol), Pd 2 (dba) 3 (147 mg, 0.16 mmol), s-Phos (193 mg, 0.47 mmol) and t-BuONa (1.2 g, 12.52 mmol) in 100 mL of toluene were heated at 110° C. overnight under argon. After cooling to room temperature, the mixture was extracted with dichloromethane. The resulting organic extract was dried over Na2SO4 and evaporated under reduced pressure. The crude product was purified by silica gel column chromatography using petroleum ether / dichloromethane (PE / DCM, 1 / 1, V / V) as eluent to obtain BPCN-Cz2Ph as a white solid (1.24 g, 80%), Figure 4-5 respectively its 1 H-NMR and 13 C-NMR spectrum, its 1 H-NMR and 13 The corresponding results of C-NMR are as follows:
[0062] BPCN-Cz2Ph: 1 H NMR (600MHz, DMSO) δ8.77 (s, 2H), 8.04 (d, J = 7.8Hz, 1H), 7.93 (d, J = 7.8Hz, 2H), 7.90–7.85 (m, 3H), 7.83 (d, J=8.1Hz, 5H), 7.80(d, J=11.6Hz, 2H), 7.66(t, J=7.6Hz, 1H), 7.57(d, J=8.6H...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
| luminescence spectroscopy | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com