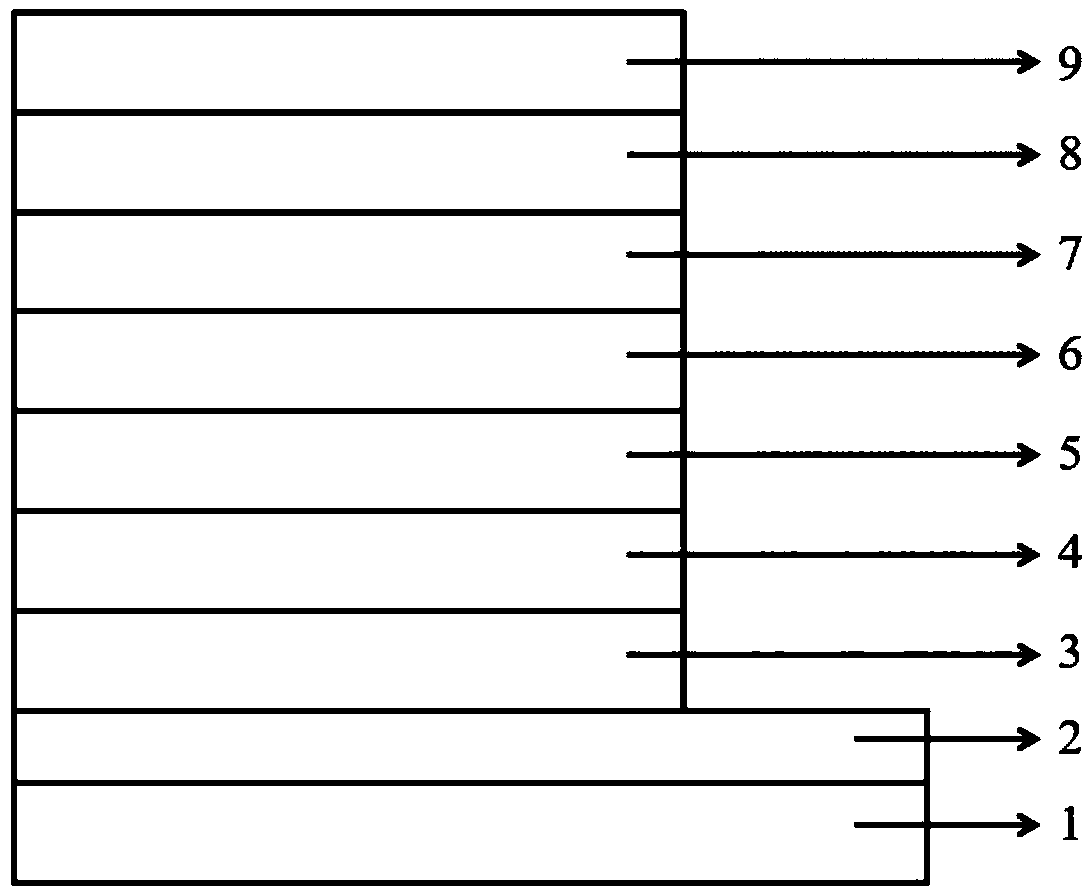
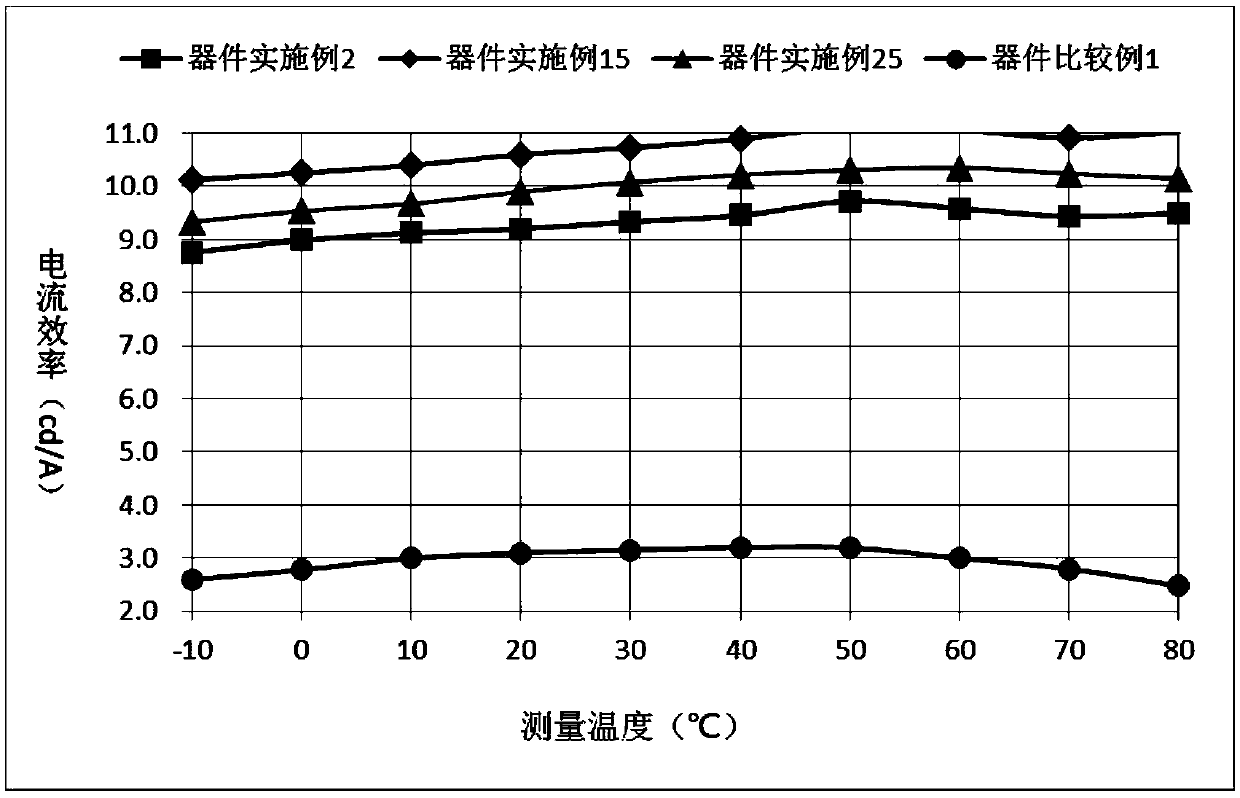
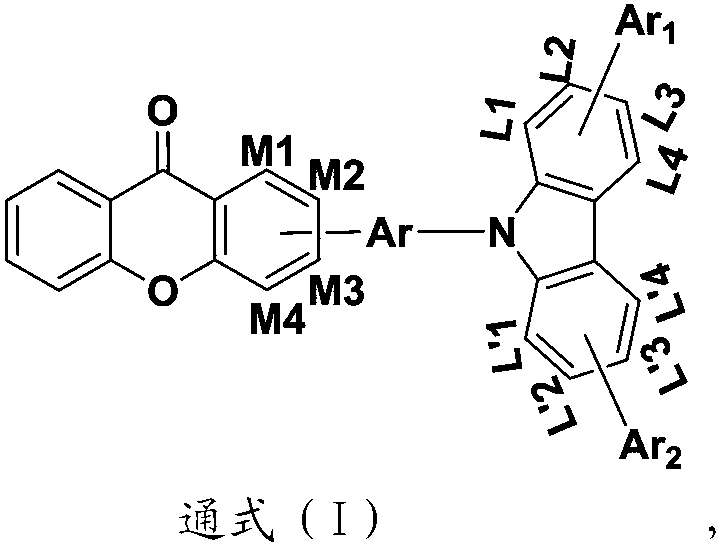
Heterocyclic compound with xanthone as core and preparation method and applications thereof
A technology of heterocyclic compounds and xanthone, which is applied in the field of heterocyclic compounds, can solve the problems of low S1 state radiation transition rate, difficult high exciton utilization rate, high fluorescence radiation efficiency, and efficiency roll-off, etc., to achieve enhanced luminescence Effects of efficiency and service life, polymer thermal stability, and large steric hindrance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0267] Preparation of Compound S13
[0268]
[0269] 1) Under a nitrogen atmosphere, add 0.1mol of raw material D-1 and 0.3mol of raw material E-1 to a 250mL three-neck flask, add 100mL of 1,4-dioxane to dissolve them, and stir for 30min with nitrogen gas, then add 0.02mol CuI, 0.02mol trans-1,2-diaminocyclohexane, 0.4mol K 3 PO 4 , heated to 110° C., reacted for 24 h, and observed the reaction by thin-layer chromatography (TLC) until the reaction was complete. After naturally cooling to room temperature, NH 4 CO 3 The aqueous solution was extracted with dichloromethane, separated, and the organic phase was rotary evaporated under reduced pressure until there was no fraction. The obtained substance was purified by silica gel column to obtain intermediate F-1 with a purity of 99.8% and a yield of 71.6%.
[0270] Elemental analysis structure (molecular formula C 43 h 29 N 3 ): theoretical value C, 87.88; H, 4.97; N, 7.15; test value: C, 87.86; H, 4.98; N, 7.16. ESI-...
preparation Embodiment 2
[0275] Preparation of Compound S19
[0276]
[0277] 1) Under a nitrogen atmosphere, add 0.1mol of raw material D-1 and 0.3mol of raw material E-1 to a 250mL three-neck flask, add 100mL of 1,4-dioxane to dissolve them, and stir for 30min with nitrogen gas, then add 0.02mol CuI, 0.02mol trans-1,2-diaminocyclohexane, 0.4mol K 3 PO 4 , heated to 110° C., reacted for 24 h, and observed the reaction by thin-layer chromatography (TLC) until the reaction was complete. After naturally cooling to room temperature, NH 4 CO 3 The aqueous solution was extracted with dichloromethane, separated, and the organic phase was rotary evaporated under reduced pressure until there was no fraction. The obtained substance was purified by silica gel column to obtain intermediate F-1 with a purity of 99.8% and a yield of 71.6%.
[0278] Elemental analysis structure (molecular formula C 43 h 29 N 3 ): theoretical value C, 87.88; H, 4.97; N, 7.15; test value: C, 87.86; H, 4.98; N, 7.16. ESI-...
preparation Embodiment 3
[0287] Preparation of Compound S39
[0288]
[0289] 1) Under a nitrogen atmosphere, add 0.2 mol of the prepared intermediate II-1, 0.3 mol of raw material IV-2, 0.15 mol of sodium tert-butoxide, 1×10 -4 mol Pd 2 (dba) 3 and 1×10 -4 mol of tri-tert-butylphosphine, then add 150mL of toluene to dissolve it, heat to 100°C, reflux for 24h, observe the reaction by TLC until the reaction is complete. Naturally cooled to room temperature, filtered, and the filtrate was rotary evaporated until there was no fraction. The obtained substance was purified by silica gel column to obtain intermediate A-2 with a purity of 99.3% and a yield of 75.9%.
[0290] Elemental analysis structure (molecular formula C 44 h 30 BrN 3 ): Theoretical C, 77.64; H, 4.44; Br, 11.74; N, 6.17; Found: C, 77.65; H, 4.45; Br, 11.75; N, 6.15. ESI-MS(m / z)(M + ): The theoretical value is 679.16, and the measured value is 679.46.
[0291] 2) Under a nitrogen atmosphere, add 0.1mol of intermediate A-2 to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com