Foot-and-mouth disease virus-like particle antigen, vaccine composition prepared therefrom, and preparation method and application thereof
A technology of foot-and-mouth disease virus and vaccine composition, which is applied in the field of veterinary biological products, can solve the problems of inability to protect against various topological O-type foot-and-mouth disease viruses, inability to adapt to the healthy development of animal husbandry, and inability to completely eliminate FMDV. Biosafety risk, good immune efficacy, low production cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment approach
[0023] As an embodiment of the present invention, the pharmaceutically acceptable carrier includes an adjuvant, and the adjuvant includes: (1) aluminum glue adjuvant, saponin, avridine, DDA; (2) water-in-oil emulsion , oil-in-water emulsions, water-in-oil-in-water emulsions; or (3) polymers of acrylic or methacrylic acid, copolymers of maleic anhydride and alkenyl derivatives; and RIBI adjuvant systems, Block co- One or more of polymer, SAF-M, monophosphoryl lipid A, Avridine lipid-amine adjuvant, E. coli heat labile enterotoxin, cholera toxin, IMS 1314, muramyl dipeptide, and Gel adjuvant kind;
[0024] Preferably, the saponin is Quil A, QS-21, GPI-0100;
[0025] Preferably, the emulsion is an SPT emulsion, a MF59 emulsion, or the emulsion is formed by combining an oil with an emulsifier, and the emulsion may be based on light liquid paraffin oil, isoprenoid oil (such as squalane or squalene) produced by oligomerization of olefins Oils, olefins, especially oils resulting fr...
Embodiment 1
[0041]Example 1 Expression of foot-and-mouth disease virus-like particles
[0042] 1. Construction of Expression Vectors
[0043] The VP4 gene fragment shown in SEQ ID NO.1 in the sequence listing, the VP2 gene fragment shown in the sequence listing SEQ ID NO.2, the VP3 gene fragment shown in the sequence listing SEQ ID NO.3, and the SEQ ID NO.3 in the sequence listing were synthesized by Jinweizhi Company. The VP1 gene fragments shown in ID NO.4 were respectively connected with the pBLUE-T Vector vector, and the successfully connected recombinant clones were respectively treated with BamH I / EcoR I, PstI / XbaI, Sac I / Sal I, Hind III / Xho I enzymes The digested fragment was ligated with the pET28a vector digested by the same enzyme to obtain a positive clone pET28a-VP4-VP2-VP3-VP1 inserted into the VP4, VP2, VP3 and VP1 genes of the O-type foot-and-mouth disease virus. Transform the ligated plasmid into CaCl 2 The prepared DH5α competent cells were spread on kanamycin-resistant...
Embodiment 2
[0051] Example 2 Preparation of foot-and-mouth disease virus-like particle vaccine composition
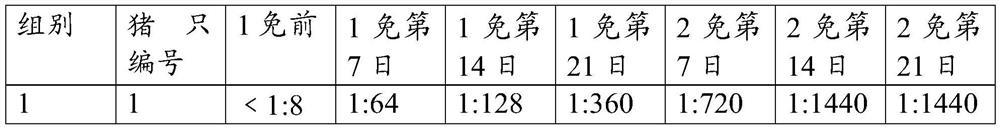
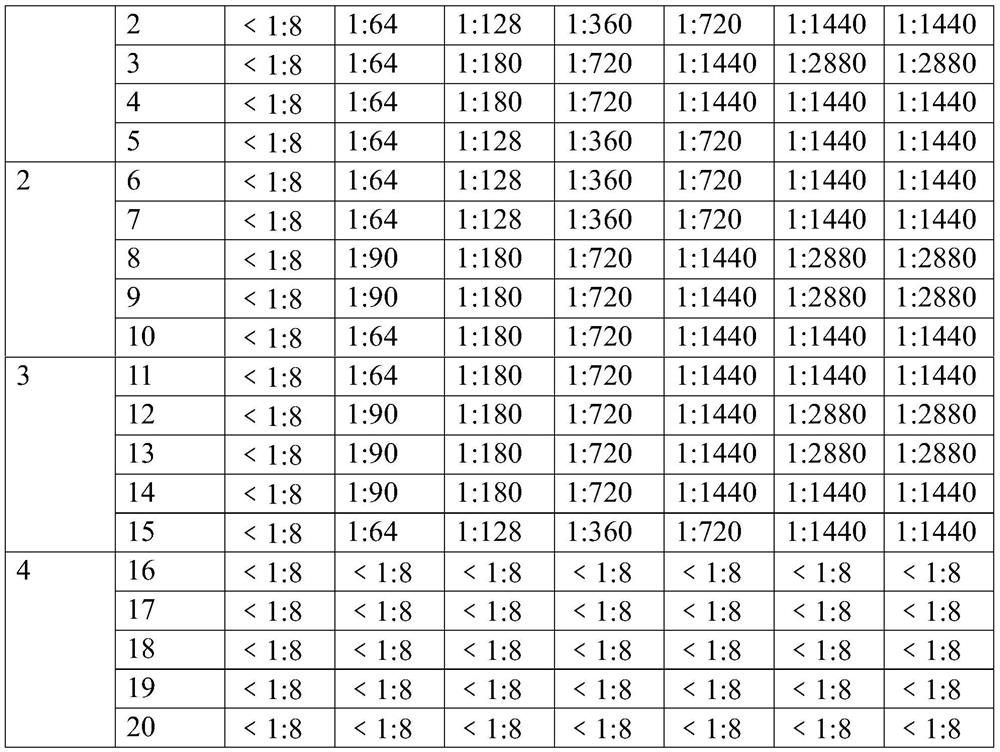
[0052] The virus-like particles prepared in Example 1 were slowly added to the adjuvant. During the addition, the mixture was continuously stirred for 12 minutes with an emulsifier with a rotating speed of 800 rpm, mixed well, and stored at 4°C, which was the foot-and-mouth disease virus-like particle vaccine composition. The specific ratio is shown in Table 1. Adjuvants suitable for use in the present invention may be adjuvants known to those skilled in the art. In the present example, the adjuvant ISA206 (France Saibiq) was selected.
[0053] Table 1 Composition ratio of foot-and-mouth disease virus-like particle vaccine composition
[0054] Vaccine 1 Vaccine 2 Vaccine 3 Foot and mouth disease antigen (μg / ml) 160 200 240 ISA 206 adjuvant (V / V%) 50% 50% 50%
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com