Silver electrode activating method
An activation method, silver electrode technology, applied in the direction of electrodes, electrode shape/type, electrolysis process, etc., can solve the problems of low surface area of three-dimensional porous structure, easy collapse of three-dimensional porous structure, low removal rate of miscellaneous metals, etc. The effect of improving dechlorination activity and high removal rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1: the activation method of bright silver electrode
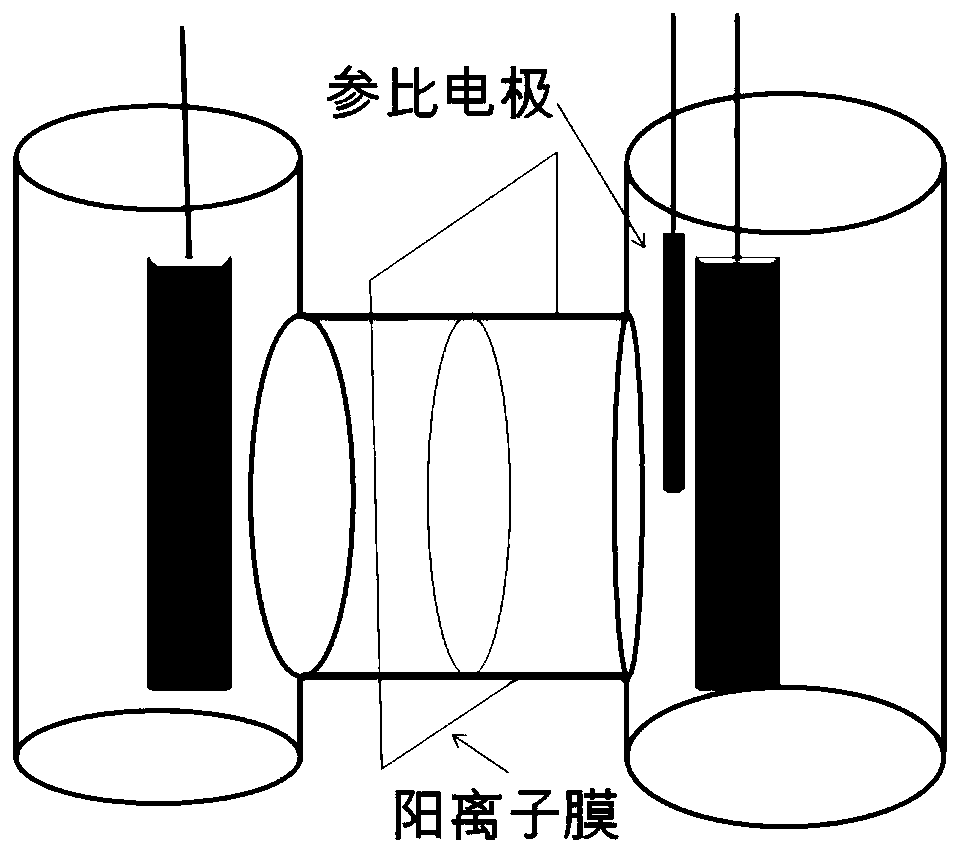
[0034] (1) Redox of acid solution: in H-type cationic membrane electrolyzer with Nafion 117 cationic membrane (such as figure 1Shown), bright silver sheet (purity is 99.9wt%, size is 0.1cm * 2.0cm * 2.0cm) is working electrode; Platinum sheet of the same area is counter electrode; Silver / silver chloride is reference electrode. The working electrode chamber is a 10wt% hydrochloric acid aqueous solution (referred to as 1# electrolyte), and the counter electrode chamber is a 1.0mol / L sodium hydroxide aqueous solution. Control the temperature of the aqueous hydrochloric acid solution at 20-25°C, first apply a current density of 0.5A / dm to the silver electrode 2 The anodizing current until the electrode potential reaches +0.7vs.SHE; then reverse the current (with the silver sheet as the counter electrode and the platinum sheet as the working electrode), and apply a current density of 0.5A / dm to the silver elec...
Embodiment 2-7
[0037] According to the method of embodiment 1, change 1# electrolytic solution and 2# electrolytic solution in embodiment 1, redox current density, redox cut-off potential and reaction temperature into table 1, and the activated silver roughness prepared is shown in table 1 .
[0038] Table 1 Experimental parameters and roughness of activated silver electrode
[0039]
Embodiment 8
[0044] Embodiment 8: the electrochemical dechlorination of dichloromethane (silver electrode is not activated in the middle of the batch reaction)
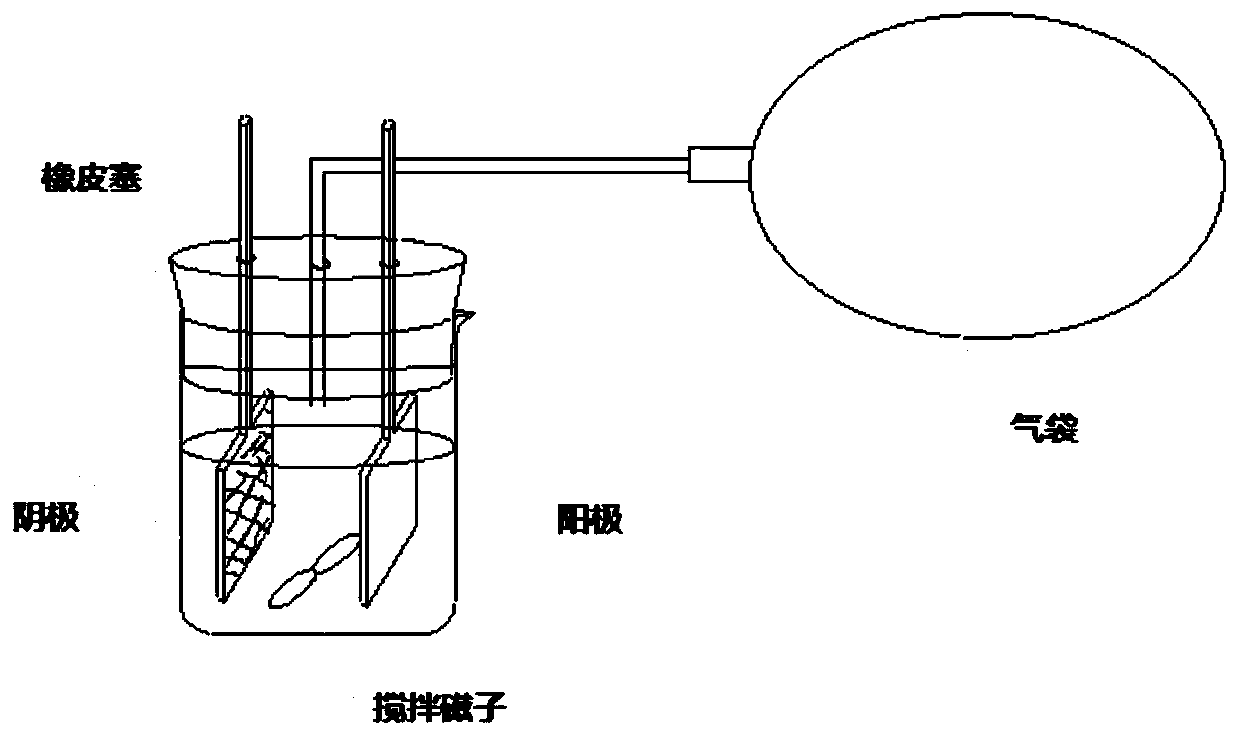
[0045] Sealed diaphragmless electrolyzers with air pockets (e.g. figure 2 Shown), the activated silver electrode (geometric dimension is 0.1cm * 2.0cm * 2.0cm, purity is 99.9wt%) prepared by the method described in Example 1 is the negative electrode, and the magnesium plate of the same area (geometric dimension is 0.3cm ×2.0cm×2.0cm) is the anode; 50mL of acetonitrile solution containing 0.1mol / L tetrabutylammonium perchlorate+5wt% acetic acid+20mmol / L dichloromethane is the electrolyte. At 25-30°C, feed 1A / dm 2 After 4 hours of reaction, the electrolysis was stopped. The yield of methane determined by gas chromatography is: 98%; the conversion rate of dichloromethane is 100%.
[0046] After repeating the above-mentioned electrochemical dechlorination process of dichloromethane 5 times, the roughness of the activated silver e...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com