Fenton reagent and adriamycin co-transport targeting nanocarrier and preparation method thereof
A Fenton’s reagent and nanocarrier technology, which is applied in the direction of pharmaceutical formulations, medical preparations with non-active ingredients, medical preparations containing active ingredients, etc., can solve the problem of co-transportation of Fenton’s reagent and doxorubicin. Carrier preparation method and other issues, to achieve stable release rate, high encapsulation rate and drug loading rate, and fast release rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
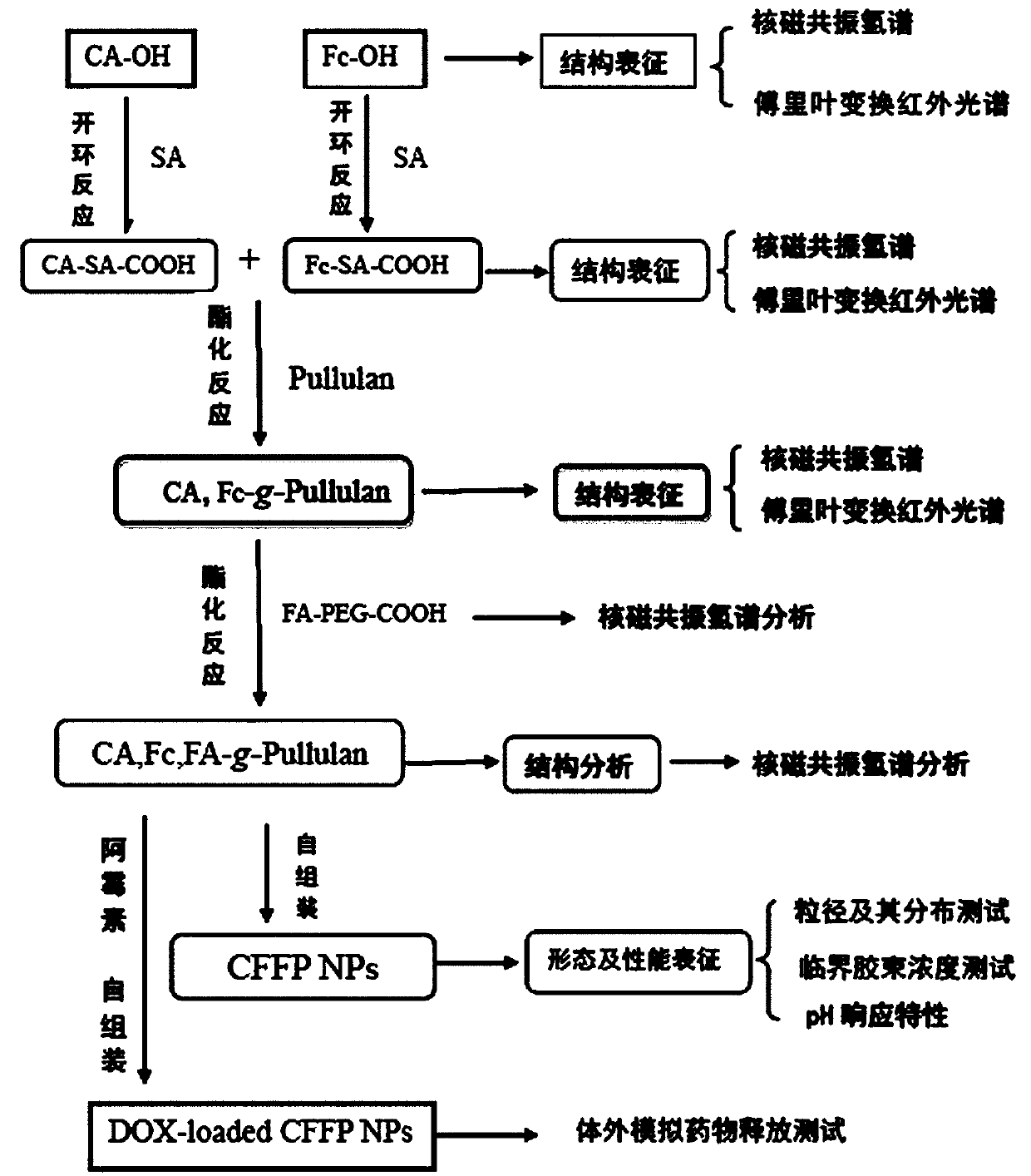
Embodiment 1
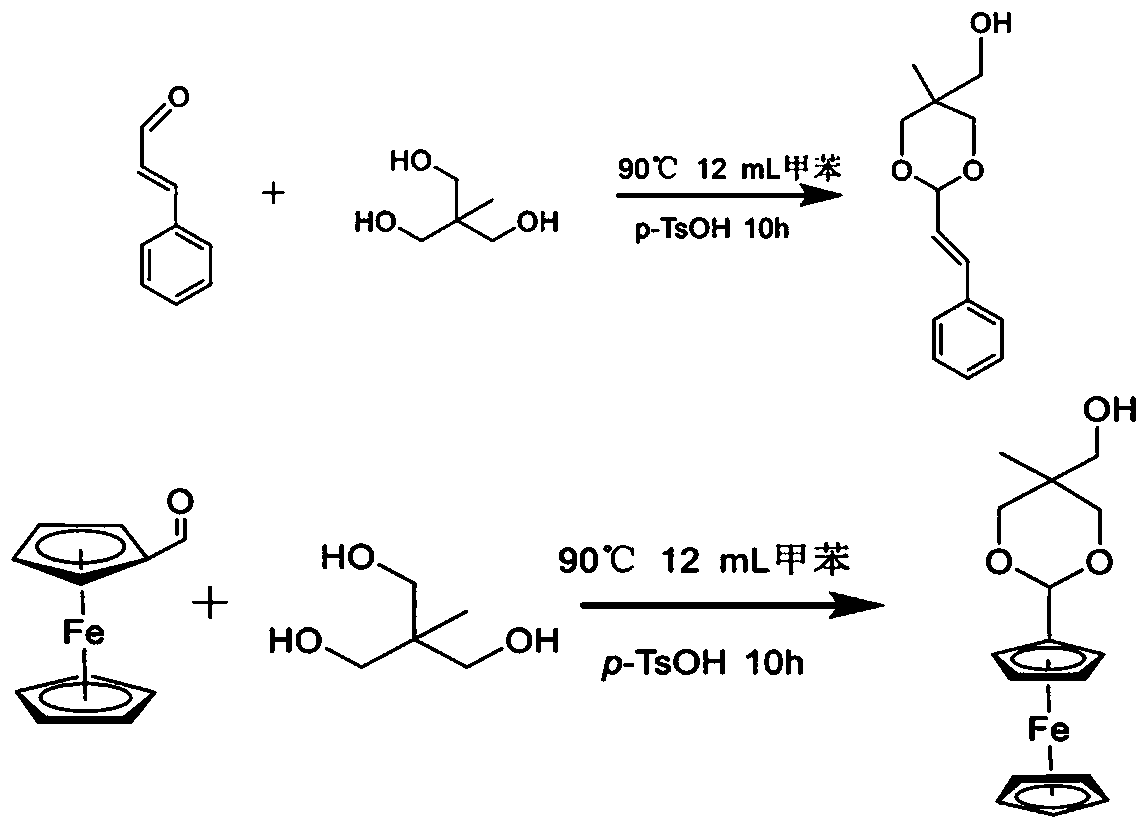
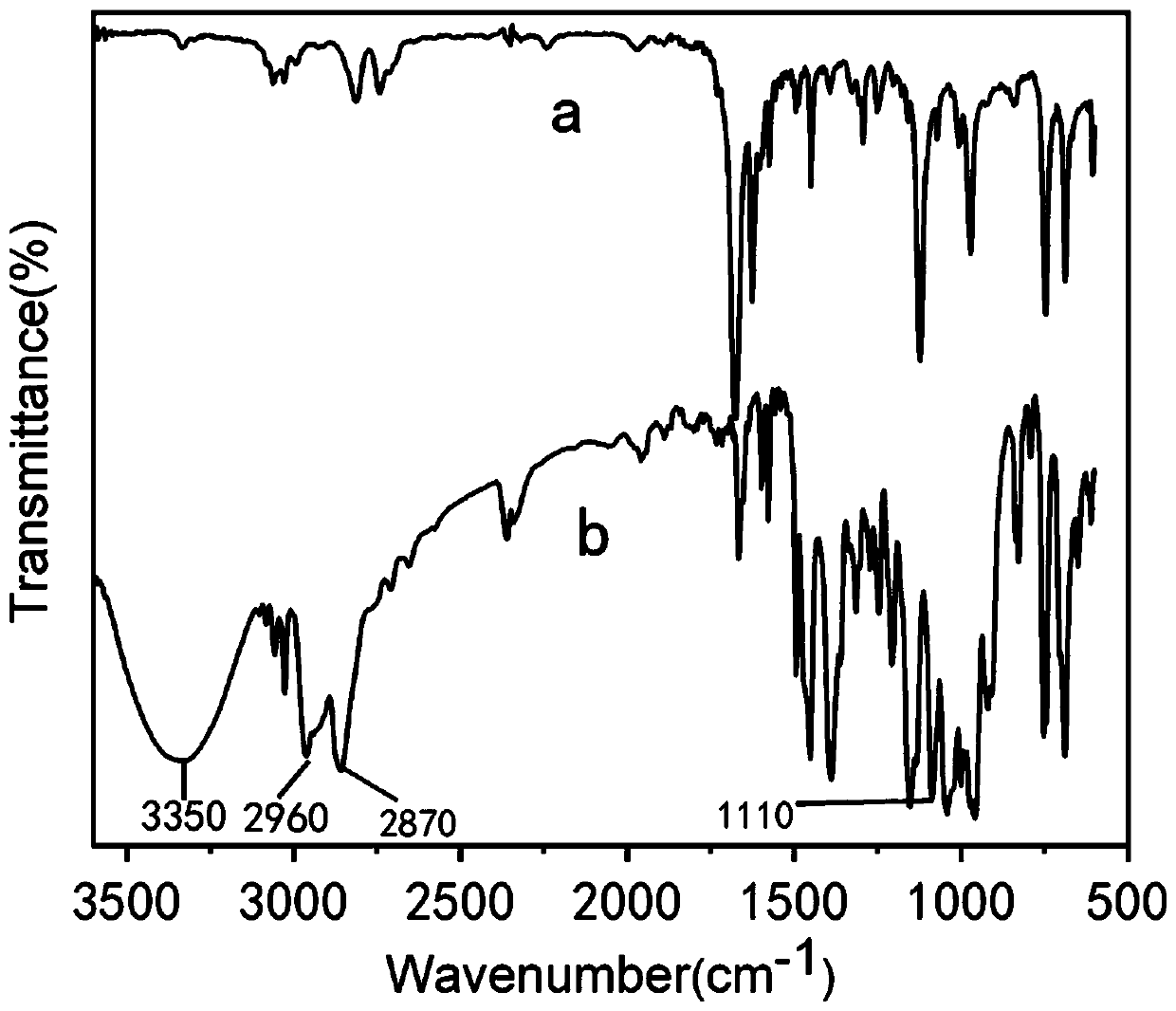
[0062] The preparation of embodiment 1 CA-OH and Fc-OH
[0063] Preparation of CA-OH:
[0064] 1) In a 100 mL eggplant-shaped bottle, dissolve 12 mmol cinnamaldehyde and 15.6 mmol trimethylolethane in 12 mL toluene, and dissolve the above raw materials in a magnetic stirring oil bath.
[0065] 2) After the raw materials are completely dissolved, add 0.12 mmol catalyst p -TsOH, stirred and reacted at 90° C. for 10 h.
[0066] 3) After the reaction, toluene was removed by rotary evaporation at 45°C to obtain a yellow viscous substance, namely the product acetal;
[0067] with dichloromethane (CH 2 Cl 2 ) After dissolving the yellow sticky substance completely, add an appropriate amount of 200-300 mesh chromatographic silica gel powder to mix the sample, and add 3 drops of triethylamine during the sample mixing process to prevent the acetal decomposition of the product.
[0068] 4) Carry out column chromatography separation and purification
[0069] The column press agent i...
Embodiment 2
[0082] The preparation of embodiment 2CA-SA-COOH and Fc-SA-COOH
[0083] Preparation of CA-SA-COOH:
[0084] 1) In a 100 mL eggplant-shaped flask, 2.15 mmol of CA-OH synthesized in Example 1 and 3.23 mmol of dry succinic anhydride were dissolved in 5 mL of butanone, and the raw materials were dissolved in a magnetic stirring oil bath.
[0085]2) After the raw materials are completely dissolved, add 0.5 mL acid-binding agent triethylamine (TEA), condense and reflux, and stir the reaction at 80°C for 12 hours.
[0086] 3) After the reaction, butanone was removed by rotary evaporation at 40°C, and the reaction solution was mixed with silica gel powder into powder, and 3 drops of triethylamine were added during the sample mixing process.
[0087] 4) Carry out column chromatography separation and purification
[0088] The pressure column agent is made of petroleum ether and triethylamine in a volume ratio of 60:1, and the eluent is CH 2 Cl 2 、CH 3 OH was compounded at a volume...
Embodiment 3
[0101] The preparation of embodiment 3FA-PEG-COOH
[0102] 1) In a 50 mL eggplant-shaped bottle, mix 0.258 mmol FA·H 2 O and 0.258 mmol EDC·HCl were dissolved in 10 mL DMSO and stirred on a magnetic stirrer for 3 h to activate the g-carboxyl group of FA molecules.
[0103] 2) The activated FA solution was added dropwise to a solution containing 0.172 mmol NH 2 -PEG-COOH in 5 mL DMSO solution, with strong magnetic stirring drop by drop, and stirred at 65 °C for 24 h.
[0104] 3) After the reaction, the reaction solution was dialyzed in DMSO with an average molecular weight cut-off of 300 for 12 hours to remove excess FA molecules, and then dialyzed with distilled water for one day (changing the water several times) to remove the product of the activator EDC and DMSO.
[0105] 4) Finally, 0.3543 g of yellow solid FA-PEG-COOH was obtained by freeze-drying, with a yield of 85%.
[0106] Example 3 The reaction equation for the synthesis of FA-PEG-COOH is attached Figure 12 sho...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| cumulative release rate | aaaaa | aaaaa |
| cumulative release rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com