Synthesis method of (E)-beta-aryl-beta,gamma-unsaturated ester compound
A technology for ester compounds and synthesis methods, which is applied in the preparation of organic compounds, organic chemistry methods, chemical instruments and methods, etc., can solve the problems of difficult and easy control of stereoselectivity, difficult operation of gas raw materials, and the need for metal catalysts. Wide range of compounds, cheap reagents, and high yields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
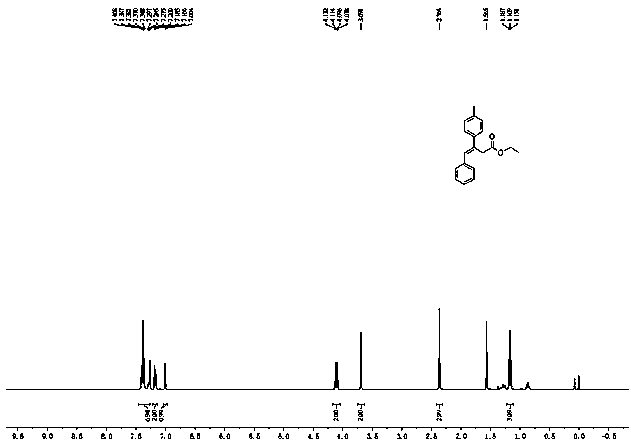
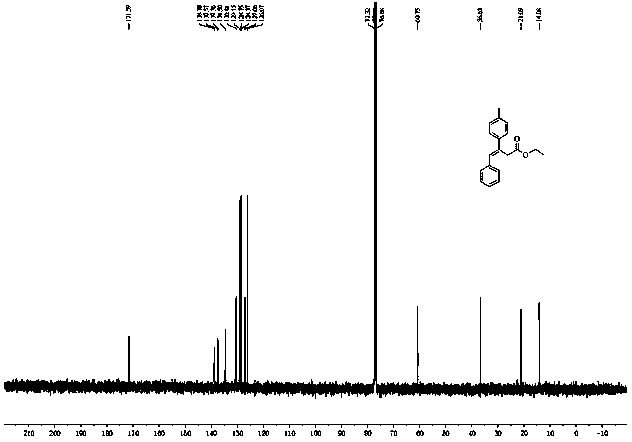
[0029] First, put a stir bar in a 35 mL sealed tube, and add 33 μL ethyl diazoacetate (0.3 mmol), 1,4-dioxane (1 mL), 41 μL phenylacetylene (0.36 mmol ), 124.9 mg p-tolueneboronic acid (0.9 mmol), mix well. Then, 5.4 mg Phen (0.03 mmol), 5.7 mg CuI (0.03 mmol) and 130.0 mg K 3 PO 4 (0.6 mmol). After adding all the medicines, feed nitrogen into the sealed tube for about 3 minutes, remove the air in the sealed tube, seal the tube mouth with a cock, and stir and react at 100° C. for 1.5 hours. After the reaction was finished, the system was cooled to room temperature, 3 mL of distilled water was added to the reaction system, extracted with ethyl acetate, the organic phases were combined, the organic phase solvent was removed by distillation under reduced pressure, and the reaction system was subjected to silica gel column chromatography (V 石油醚 :V 乙酸乙酯 =50:1) to obtain 71.0 mg of colorless liquid product 4a, yield 85%. The reaction is shown in the following formula:
[0030...
Embodiment 2
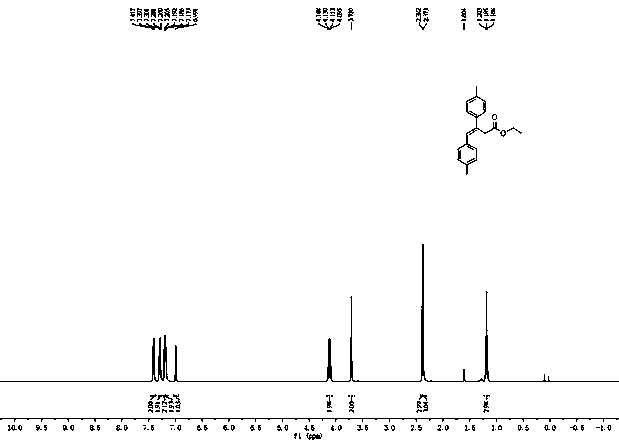
[0037] First, put a stirring bar in a 35 mL sealed tube, and add 33 μL of ethyl diazoacetate (0.3 mmol), 1,4-dioxane (1 mL), 47 μL of p-methylphenylacetylene (0.36 mmol), 124.9 mg p-tolueneboronic acid (0.9 mmol), mix well. Then, 5.4 mg Phen (0.03 mmol), 5.7 mg CuI (0.03 mmol) and 130.0 mg K 3 PO 4 (0.6 mmol). After adding all the medicines, pass nitrogen into the sealed tube for about 3 minutes, remove the air in the sealed tube, seal the mouth of the tube with a cock, and stir at 100°C for 1.5 hours. After the reaction was finished, the system was cooled to room temperature, 3 mL of distilled water was added to the reaction system, extracted with ethyl acetate, the organic phases were combined, the solvent of the organic phase was removed by distillation under reduced pressure, and the reaction system was subjected to silica gel column chromatography (V 石油醚 :V 乙酸乙酯 =50:1) to obtain 72.0 mg of colorless liquid product 4b (its CAS number is 130240-35-6), with a yield of 8...
Embodiment 3
[0042] First, put a stirring bar in a 35 mL sealed tube, and add 33 μL ethyl diazoacetate (0.3 mmol), 1,4-dioxane (1 mL), 42 μL p-fluorophenylacetylene ( 0.36 mmol), 124.9 mg p-tolueneboronic acid (0.9 mmol), mix well. Then, 5.4 mg Phen (0.03 mmol), 5.7 mg CuI (0.03 mmol) and 130.0 mg K 3 PO 4 (0.6 mmol). After adding all the medicines, pass nitrogen into the sealed tube for about 3 minutes, remove the air in the sealed tube, seal the mouth of the tube with a cock, and stir at 100°C for 1.5 hours. After the reaction was finished, the system was cooled to room temperature, 3 mL of distilled water was added to the reaction system, extracted with ethyl acetate, the organic phases were combined, the solvent of the organic phase was removed by distillation under reduced pressure, and the reaction system was subjected to silica gel column chromatography (V 石油醚 :V 乙酸乙酯 =50:1) to obtain 63.0 mg of colorless liquid product 4c, yield 70%. The reaction is shown in the following for...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com