Multi-layer structured composite electrolyte and secondary battery using same
A composite electrolyte and multilayer composite technology, which is applied in the field of composite electrolyte with multilayer structure and the secondary battery using it, can solve the problem that it is difficult to find polymers or ceramics at the same time, and achieve the effect of excellent thermal stability and excellent capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
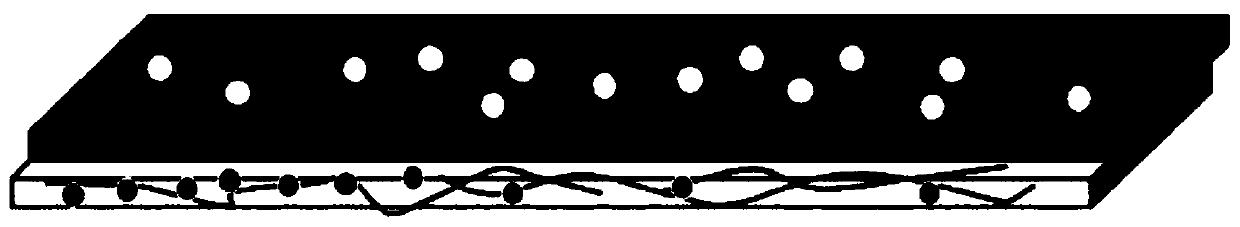
[0058] In the first composite electrolyte layer, LTAP was used as the ceramic material, PVdF was used as the polymer, and 1M LiPF in EC / DMC (ethyl carbonate / dimethyl carbonate, 1:1vol) 6 As a liquid electrolyte, in the second composite electrolyte layer, LLZO is used as a ceramic material, PVdF is used as a polymer, and 1M LiPF in EC / DMC (ethyl carbonate / dimethyl carbonate, 1:1vol) 6 as a liquid electrolyte. A multilayer composite electrolyte was prepared by laminating the first composite electrolyte layer (upper layer) and the second composite electrolyte layer (lower layer). figure 2 A schematic diagram of this multilayer composite electrolyte is shown.
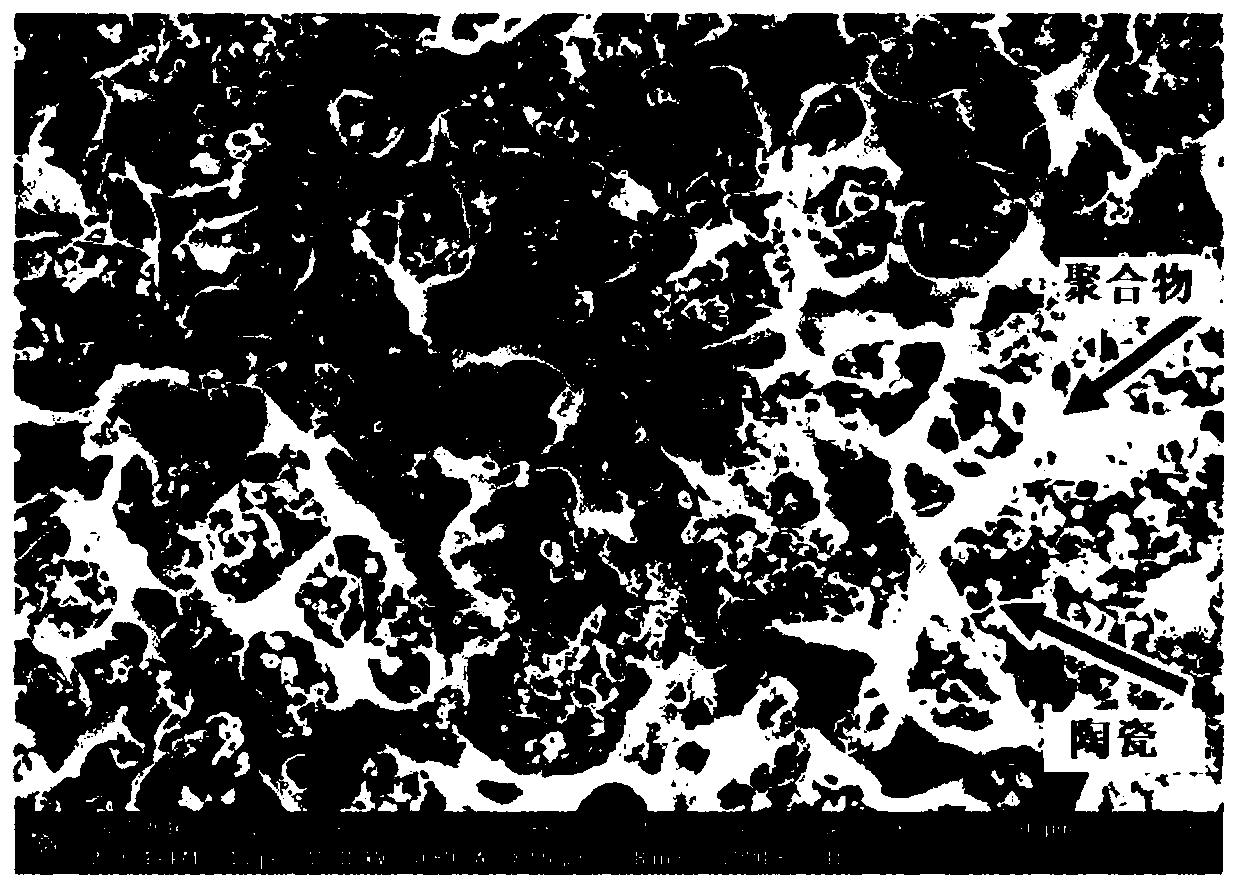
[0059] image 3 A surface SEM image of the first composite electrolyte layer is shown. Figure 4 A surface SEM image of the second composite electrolyte layer is shown. Figure 5 A cross-sectional view showing a multilayer composite electrolyte, Figure 6 The elemental analysis diagram of the section is shown.
[006...
Embodiment 2
[0062] Take LiNi 1 / 3 mn 1 / 3 co 1 / 3 o 2 (nickel-cobalt-manganese oxide lithium (NMC), lithium nickel-manganese-cobalt oxide) positive electrode material is used as positive electrode, with lithium metal as negative electrode, with the multilayer composite electrolyte of embodiment 1 as electrolyte, has prepared the secondary battery of embodiment 2 .
[0063] Use LiNi 1 / 3 mn 1 / 3 co 1 / 3 o 2 (NMC, lithium nickel manganese cobalt oxide) positive electrode material as the positive electrode, lithium metal as the negative electrode, and the first composite electrolyte layer single layer of Example 1 as the electrolyte, the secondary battery of Comparative Example 1 was prepared.
[0064] For the above two secondary batteries, charge and discharge tests were carried out under the conditions of a charge voltage of 4.4V, a discharge voltage of 3.0V, and a battery capacity of 0.1C. Figure 7 The discharge capacity showing the number of cycles of the secondary battery of Example ...
Embodiment 3
[0068] Example 3 compares the ionic conductivity and interfacial resistance according to the electrolyte. The multilayer composite electrolyte prepared in Example 1 (Example 3), the multilayer composite electrolyte prepared in Example 1 does not contain liquid electrolyte (comparative example 2), the liquid electrolyte used in Example 1 ( Comparative Example 3) for comparison.
[0069] Figure 12 The ionic conductivities at room temperature of the electrolytes of Example 3, Comparative Example 2, and Comparative Example 3 are shown. Figure 13 The interface resistances of the electrolytes of Example 3, Comparative Example 2, and Comparative Example 3 are shown. Such as Figure 12 As shown, the ionic conductivity of the liquid electrolyte of Comparative Example 3 at room temperature is 2.1×10 -3 S / cm. The multilayer composite electrolyte of embodiment 3 has an ion conductivity of 7.9×10 at room temperature -4 S / cm. The electrolyte of Comparative Example 2 (a multilayer c...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com