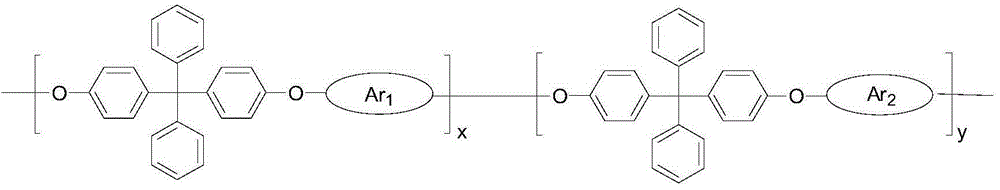
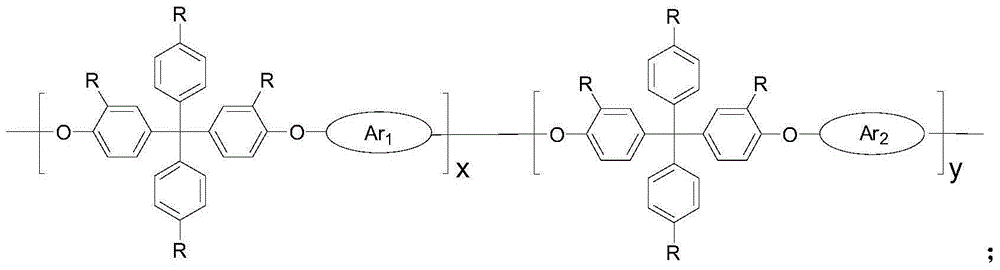
Strong Basic Polyarylether Ionomer and Its Preparation and Application
A polyarylene ether, strong alkaline technology, applied in the field of anion exchange membrane materials for alkaline fuel cells, can solve the problems of low ionic conductivity, poor stability, complicated preparation, etc., achieve high ion exchange capacity, overcome the Instable properties, good thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] The present embodiment adopts following method to prepare polyarylether:
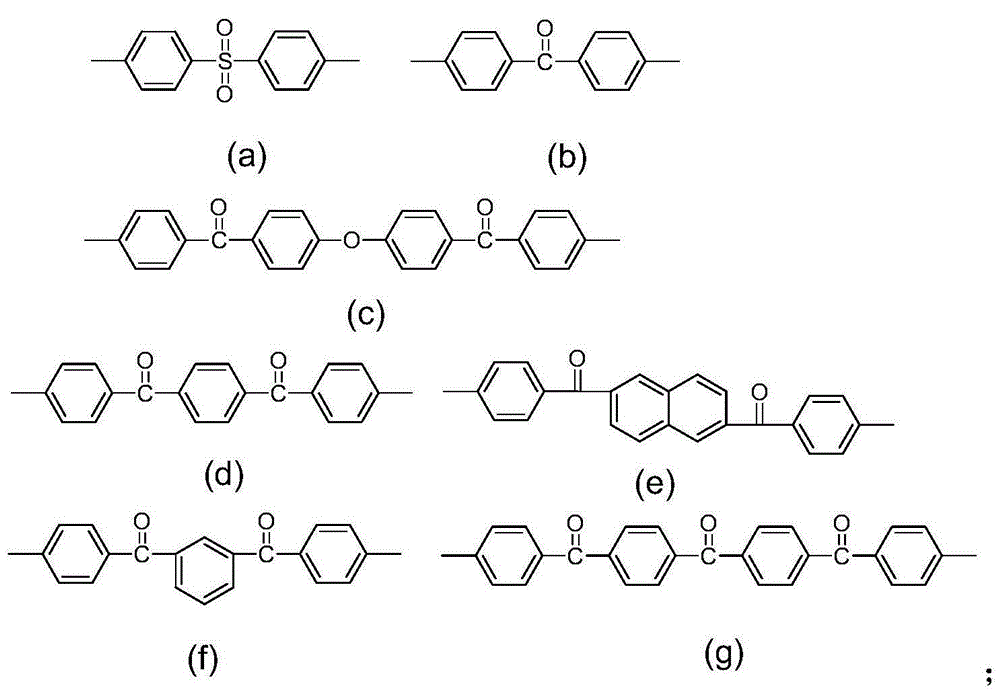
[0035] Mix bis(4,4'-hydroxyphenyl)diphenylmethane, dichlorosulfone (1-(4-chlorophenylsulfonyl)-4-chlorobenzene), difluoroketone (bis(4-fluorophenyl)methanone) at 1:0.5 : The molar ratio of 0.5 is fed, with N,N-dimethylacetamide as solvent and toluene as water-carrying agent, first react at 140°C for 4h, then heat up to 170°C for 4h, cool the reaction solution to room temperature, drop In methanol solution containing hydrochloric acid, a white flocculent precipitate is obtained, which is polyarylether.
[0036] Measured by nuclear magnetic resonance method, the polyarylether of the present embodiment has the following structure
[0037]
[0038] Utilize the polyarylether of the present embodiment to prepare the process of strongly basic polyarylether ionomer as follows:
[0039] (1) Chloromethylation of polyarylether: form a 5% solution of polyarylether with tetrachloroethane, add ZnCl 2 , h...
Embodiment 2
[0054] The present embodiment adopts following method to prepare polyarylether:
[0055] Bis(4,4'-hydroxyphenyl)diphenylmethane, dichlorosulfonyl (1-(4-chlorophenylsulfonyl)-4-chlorobenzene), 1,4-difluorobenzoylbenzene (1,4-Bis (4-fluorobenzoyl)benzene) is fed at a molar ratio of 1:0.5:0.5, with N,N-dimethylacetamide as solvent and toluene as water-carrying agent, react at 150°C for 4 hours, and then heat up to 180°C for reaction After 8 hours, the reaction solution was cooled to room temperature and dropped into methanol solution containing hydrochloric acid to obtain a white flocculent precipitate, which was polyarylether.
[0056] Measured by nuclear magnetic resonance method, the polyarylether of the present embodiment has the following structure
[0057]
[0058]
[0059] (1) Chloromethylation of polyarylether: Add the polyarylether prepared in this example to N,N-dimethylformamide to form a 5% solution, and the polyarylether: zinc chloride: methyl chloride The mo...
Embodiment 3
[0072] The present embodiment adopts following method to prepare polyarylether:
[0073] Bis(4,4'-hydroxyphenyl)diphenylmethane, difluoroketone (bis(4-fluorophenyl)methanone), 1,4-difluorobenzoylbenzene (1,4-Bis(4-fluorobenzoyl )benzene) is fed at a molar ratio of 1:0.5:0.5, using N-methylpyrrolidone as a solvent and toluene as a water-carrying agent, first reacting at 145°C for 4h, then raising the temperature to 175°C for 6h, then cooling the reaction solution to At room temperature, drop it into a methanol solution containing hydrochloric acid to obtain a white flocculent precipitate, which is polyarylether.
[0074] Measured by nuclear magnetic resonance method, the polyarylether of the present embodiment has the following structure
[0075]
[0076] In the process of preparing strong basic polyarylether ionomer and basic ion exchange membrane of polyarylether ionomer in this example, the molar ratio of polyarylether: zinc chloride: chloromethyl ether is =1:1: 40; The...
PUM
| Property | Measurement | Unit |
|---|---|---|
| water absorption | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
| water absorption | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com