A fluorescent probe for detecting water content in organic solvents and its preparation method and application
A technology of fluorescent probes and organic solvents, applied in the field of fluorescent probes for detecting water content in organic solvents and its preparation, achieving the effect of simple preparation methods
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Embodiment 1: Probe compound TPEB-m-NO 2 Synthesis
[0042] 1) Synthesis of Compound 1
[0043] Into in a mixture of tetrahydrofuran (60 mL) / water (10 mL). Under a nitrogen atmosphere, the mixture was stirred at 80°C for 24 hours. After cooling to room temperature, the solvent was evaporated under reduced pressure. Purification by silica gel column chromatography (eluent: dichloromethane / n-hexane=1:3, V / V) gave compound 1. Yield: 52.8%. 1 H NMR (600MHz, CDCl 3 )δ8.53 (s,1H),8.37–8.29(m,2H),8.01(t,J=8.9Hz,1H),7.75(t,J=8.3Hz,1H),7.71(t,J=5.7 Hz, 1H). 13 C NMR (150MHz, CDCl 3 )δ 135.06, 132.17, 129.73, 124.06, 123.38, 77.25, 77.04, 76.83. Calculated exact mass: m / z 334.936, MALDI TOF-MS: m / z 334.932[M] + .
[0044] 2) Compound TPEB-m-NO 2 Synthesis
[0045] 1mmol compound 1, 1.1mmol 4,4,5,5-tetramethyl-2-(4-(1,2,2-triphenylethenyl)phenyl)-1,3,2-dioxa Borolane, 0.05 mmol tetrakistriphenylphosphine palladium and 2 mmol potassium carbonate were added to a mixtu...
Embodiment 2
[0046] Embodiment 2: Probe compound TPEB-p-NO 2 Synthesis
[0047] 1) Synthesis of Compound 2
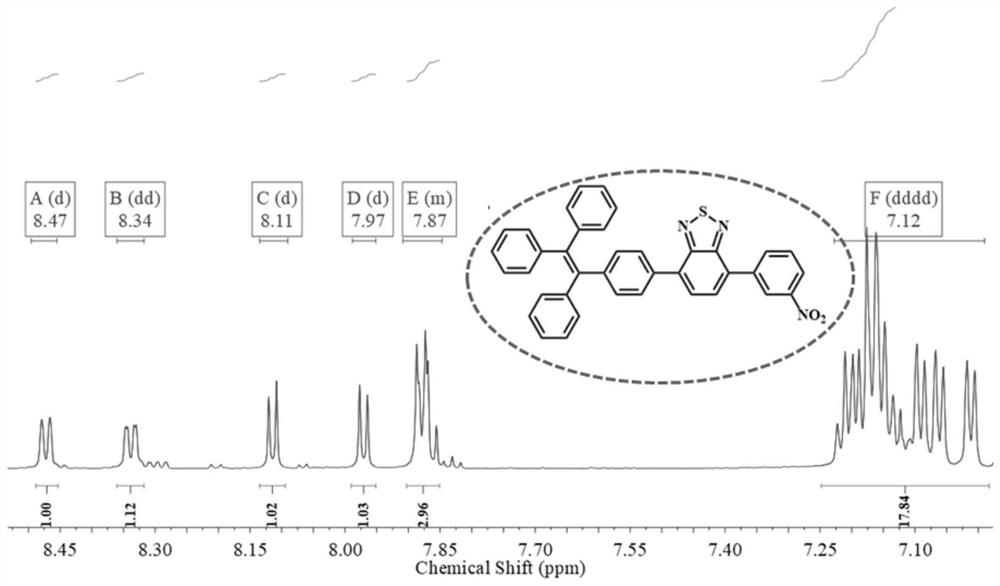
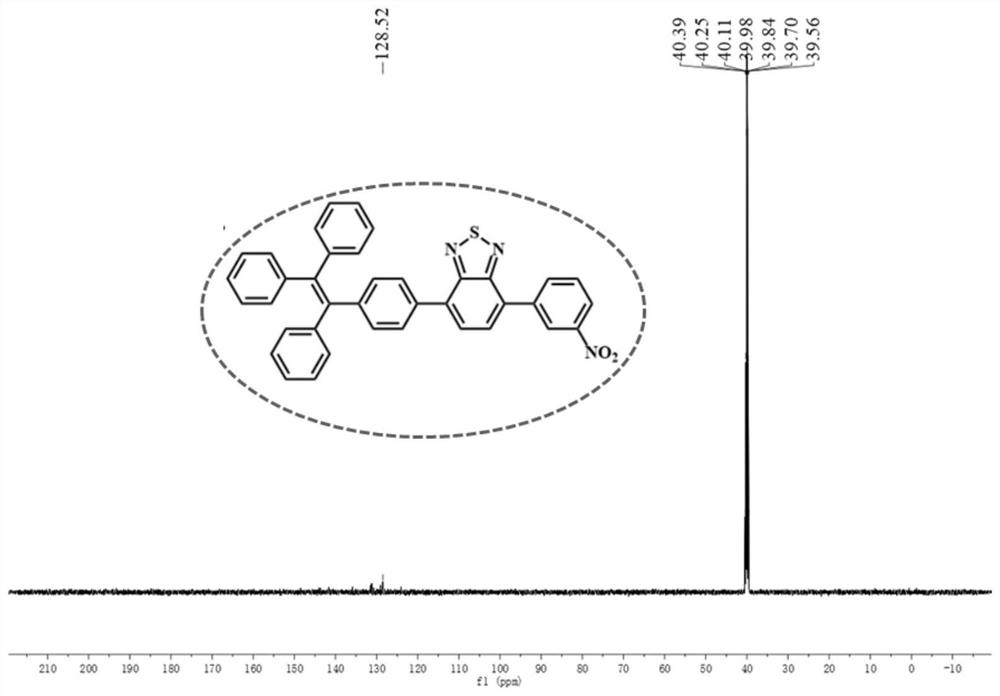
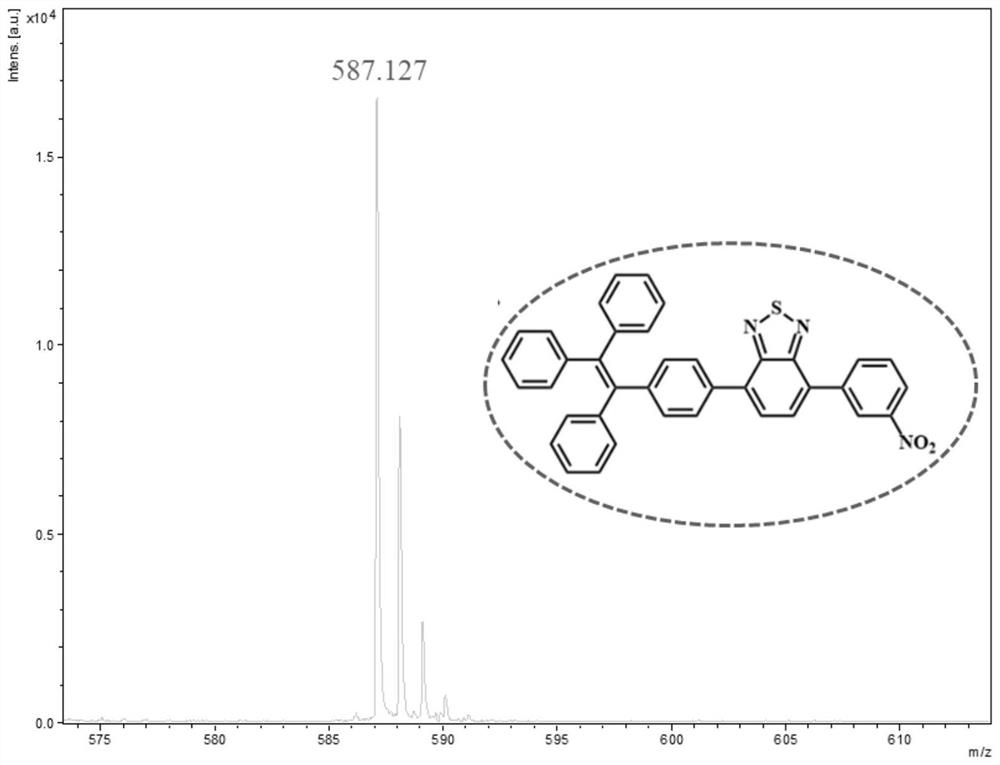
[0048] Into In a mixture of tetrahydrofuran / water (60 / 10). Under a nitrogen atmosphere, the mixture was stirred at 80°C for 24 hours. After cooling to room temperature, the solvent was evaporated under reduced pressure. Purification by silica gel column chromatography (eluent: dichloromethane / n-hexane=1:3, V / V) gave compound 2. Yield: 49.6%. 1 H NMR (600MHz, DMSO) δ8.41(d, J=8.8 Hz, 1H), 8.37(d, J=8.7Hz, 1H), 8.26(d, J=8.7Hz, 1H), 8.21(d, J =7.6Hz, 1H), 8.09(d, J=8.7Hz, 1H), 7.95(d, J=7.6Hz, 1H). 13 C NMR (150 MHz, DMSO) δ 130.81, 129.19, 124.72, 124.21, 40.39, 40.25, 40.11, 39.97, 39.83, 39.69, 39.56. Calculated exact mass: m / z 334.936, MALDI TOF-MS: m / z 335.889[M+H] + .
[0049] 2) Compound TPEB-p-NO 2 Synthesis
[0050] 1mmol compound 2, 1.1mmol 4,4,5,5-tetramethyl-2-(4-(1,2,2-triphenylethenyl)phenyl)-1,3,2-dioxa Borolane, 0.05 mmol tetrakistriphenylphosphine palladi...
Embodiment 3
[0051] Embodiment 3: In tetrahydrofuran solvent, compound TPEB-m-NO 2 and TPEB-p-NO 2 The change of fluorescence spectrum of water probe with the increase of water addition
[0052] Get the TPEB-m-NO prepared in Example 1 2 and the TPEB-p-NO prepared in Example 2 2 Water fluorescent probe, dissolved in THF, made 1 × 10 -3 mol / L stock solution. Take 30μL from the stock solution and add it to a 5mL centrifuge tube, and configure 3mL of THF / water solution with different water contents (0%, 5%, 10%, 20%, 30%, 40%, 50%, 60%, 70%, 80%, 90%) to test its fluorescence properties. Probe TPEB-m-NO 2 and TPEB-p-NO 2 The changes of fluorescence spectrum and fluorescence intensity with the increase of water content are as follows: Figure 7 to Figure 10 shown. Figure 7 with Figure 9 The insets in are respectively the probe TPEB-m-NO 2 and TPEB-p-NO 2 Emission images of water / THF solutions at different water contents, taken under 365nm UV light. For the measurement of fluores...
PUM
| Property | Measurement | Unit |
|---|---|---|
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com