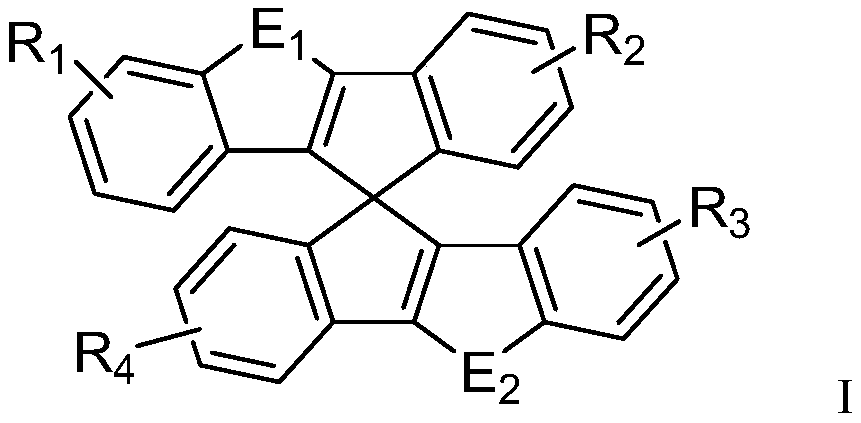
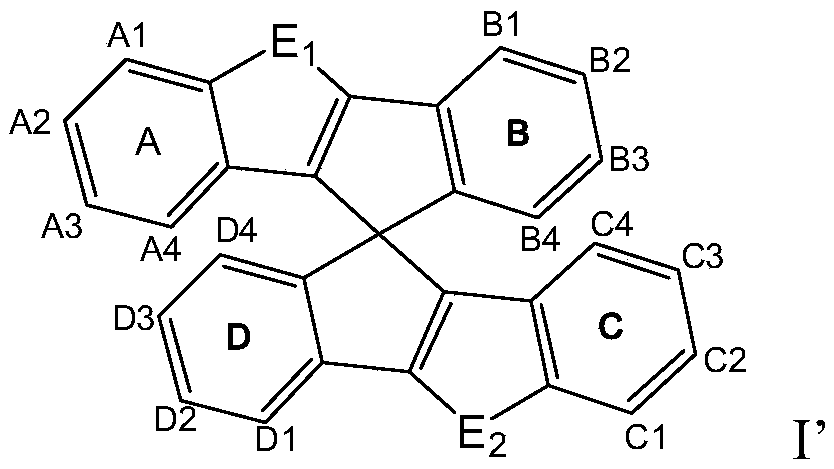
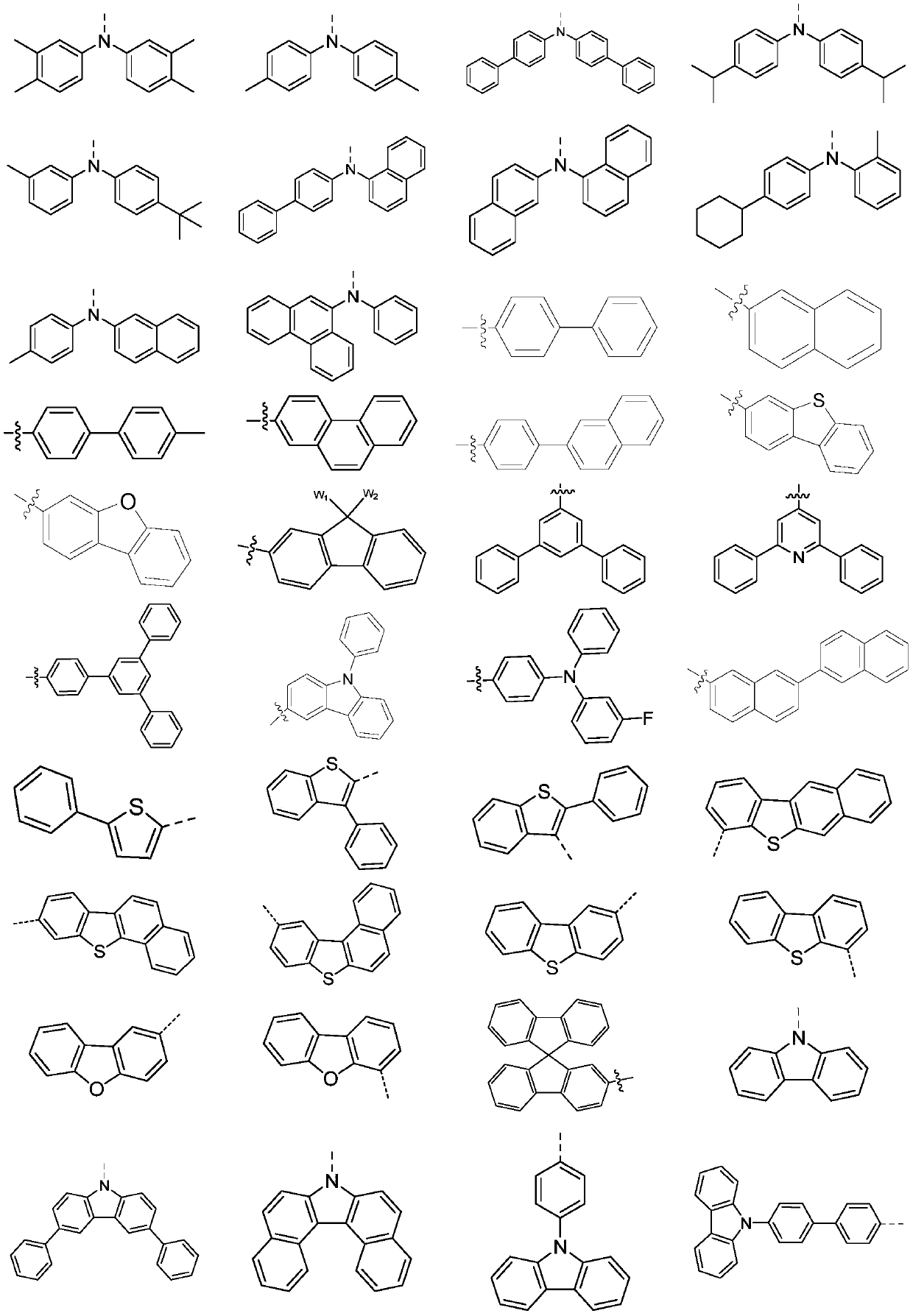
Novel heterocycle-spiro structure compound and application thereof in OLED devices
A technology of heterocyclic spiro compounds, applied in the field of organic electroluminescence display, can solve the problems of low glass transition temperature, easy crystallization of materials, damage to film uniformity, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0056] Synthesis of (Compound 1-5)
[0057] The synthetic route is as follows:
[0058]
[0059] Including the following specific steps:
[0060] A 1-liter three-necked flask equipped with a magnetic stirrer, and potassium tert-butoxide (36.2 g, 0.376 mol), bis(4-tolyl)amine (41.37 g, 0.21 mol, purity 99%) and 100 ml of toluene were added sequentially after argon replacement. After replacing with argon again, 3.4 ml of tri-tert-butylphosphine and 0.5 g of palladium acetate were successively added. After the addition was complete, the temperature was raised to 85°C. Start to drop a solution consisting of (57.0 g, 0.1 mol, purity 99%) M1 and 100 ml of toluene, and control the temperature at 80-120° C. Cool down to 50°C, add 100ml of deionized water for hydrolysis, stir for 10 minutes, filter, and boil the filter cake several times with DMF, and rotary steam to obtain 68.23g of white solid with a purity of 99.5% and a yield of about 85%.
[0061] Product MS (m / e): 802.30...
Embodiment 2
[0063] Synthesis of (Compound 1-44)
[0064] The synthetic route is as follows:
[0065]
[0066] Including the following specific steps:
[0067] 1 liter three-neck flask, equipped with magnetic stirring, after argon replacement, add potassium tert-butoxide (36.2g, 0.376mol), 4-tolyl-2-naphthylamine (48.93g, 0.21mol, purity 99%) and toluene 100ml . After replacing with argon again, 3.4 ml of tri-tert-butylphosphine and 0.5 g of palladium acetate were successively added. After the addition was complete, the temperature was raised to 85°C. Start to drop a solution consisting of (63.3g, 0.1mol, purity 99%) M2 and 100ml toluene, and control the temperature at 80-120°C. Cool down to 50°C, add 100ml of deionized water for hydrolysis, stir for 10 minutes, filter, and boil the filter cake several times with DMF, and rotary steam to obtain 78.79g of white solid with a purity of 99.5% and a yield of about 84%.
[0068] Product MS (m / e): 938.22; Elemental analysis (C 63 h 42...
Embodiment 3
[0070] Synthesis of (Compound I-99)
[0071] The synthetic route is as follows:
[0072]
[0073] Including the following specific steps:
[0074] A 1-liter three-neck flask equipped with a magnetic stirrer. After argon replacement, 19.93g (0.188mol) of sodium carbonate, 9,9-dimethyl-2-boronic acid (23.8g, 0.1mol, purity 99%) and 100ml of toluene were added in sequence. After replacing with argon again, 0.23g of Pd132 was added in sequence. After the addition, the temperature was raised to 80°C. Start to drop a solution consisting of (49.1 g, 0.1 mol, purity 99%) M3 and 100 ml of toluene, and control the temperature at 75-80°C. Cool down to room temperature, add 100ml of deionized water for hydrolysis, stir for 10 minutes, filter, and boil the filter cake several times with DMF to obtain 51.34g of white solid with a purity of 99.5% and a yield of about 85%.
[0075] Product MS (m / e): 588.21; Elemental analysis (C 44 h 28 o 2 ): theoretical value C: 89.77%, H: 4.79%...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com