Method for stably amplifying high-purity and high-cytotoxic-activity NK cells in vitro
A NK cell, high-purity technology, used in cell culture active agents, animal cells, vertebrate cells, etc., can solve the problems of weak killing function, low NK cell purity, unstable culture system, etc., and achieve high cytotoxic activity, The effect of reducing the number of
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
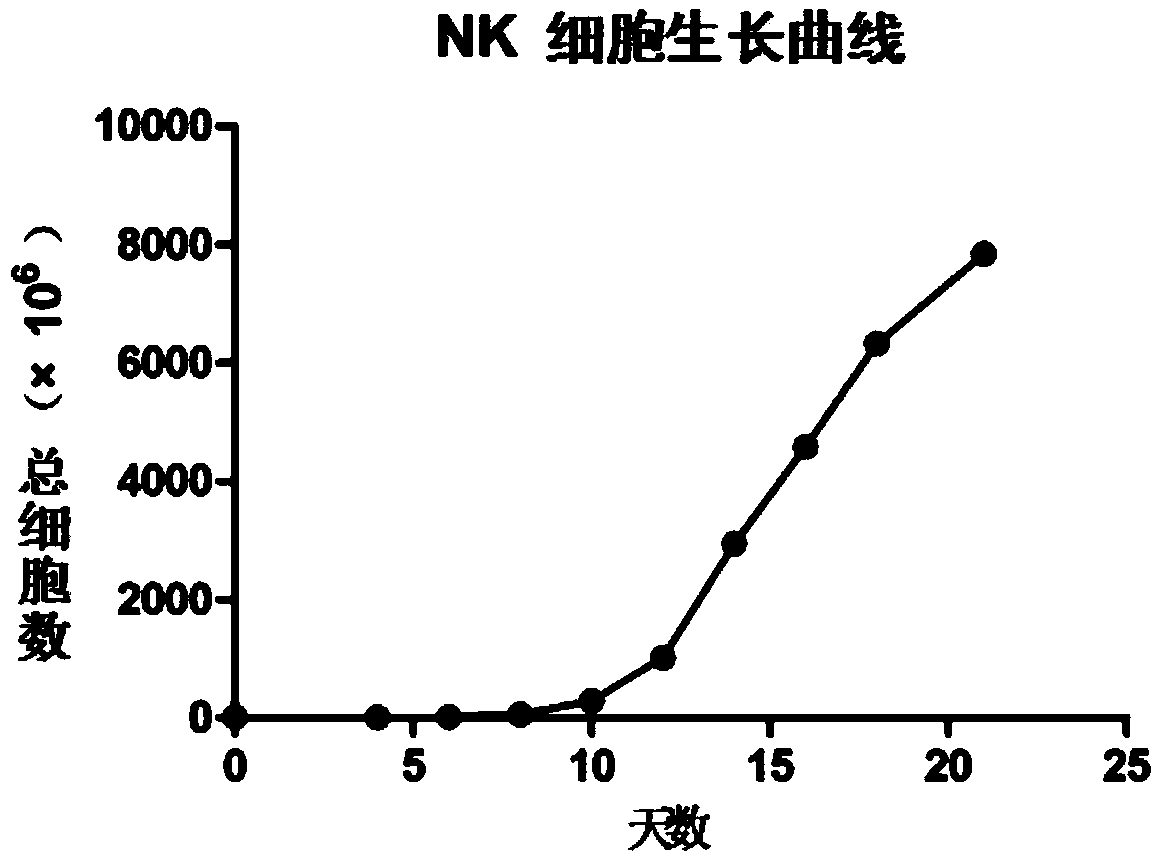
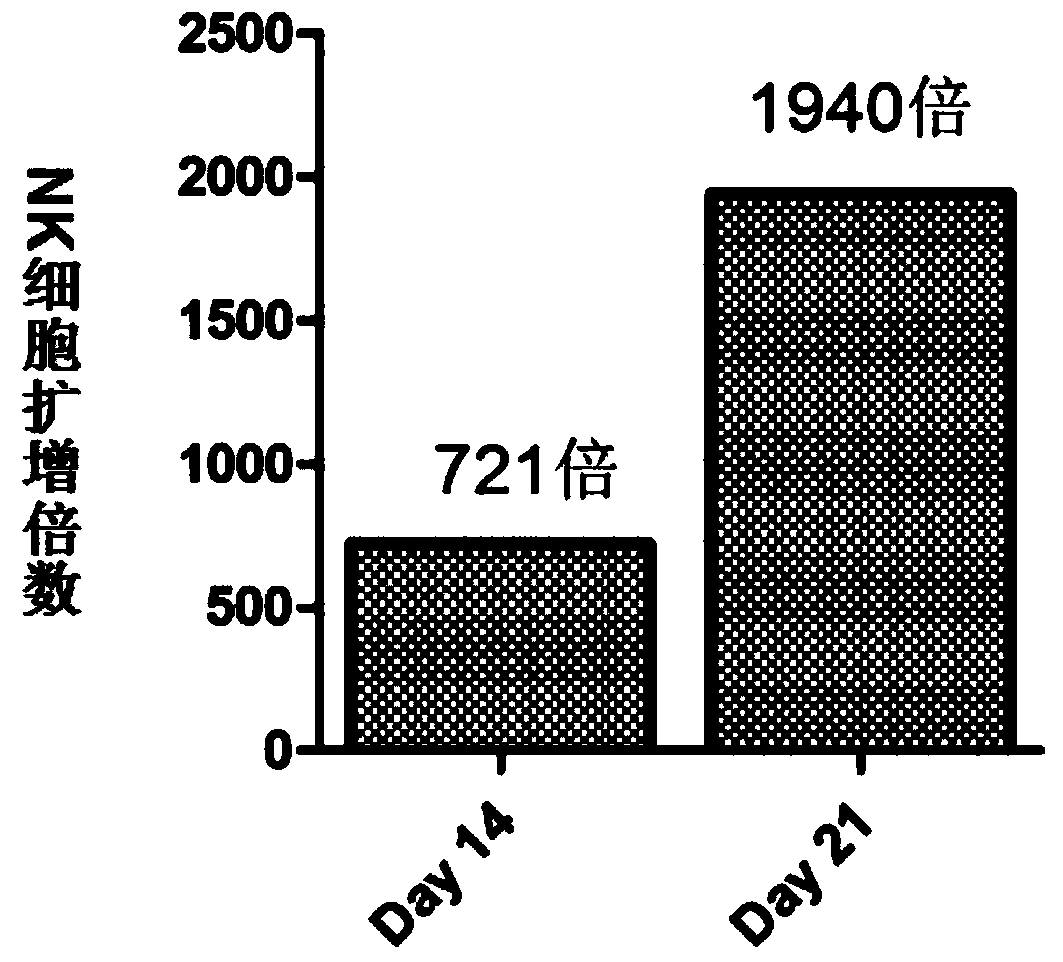
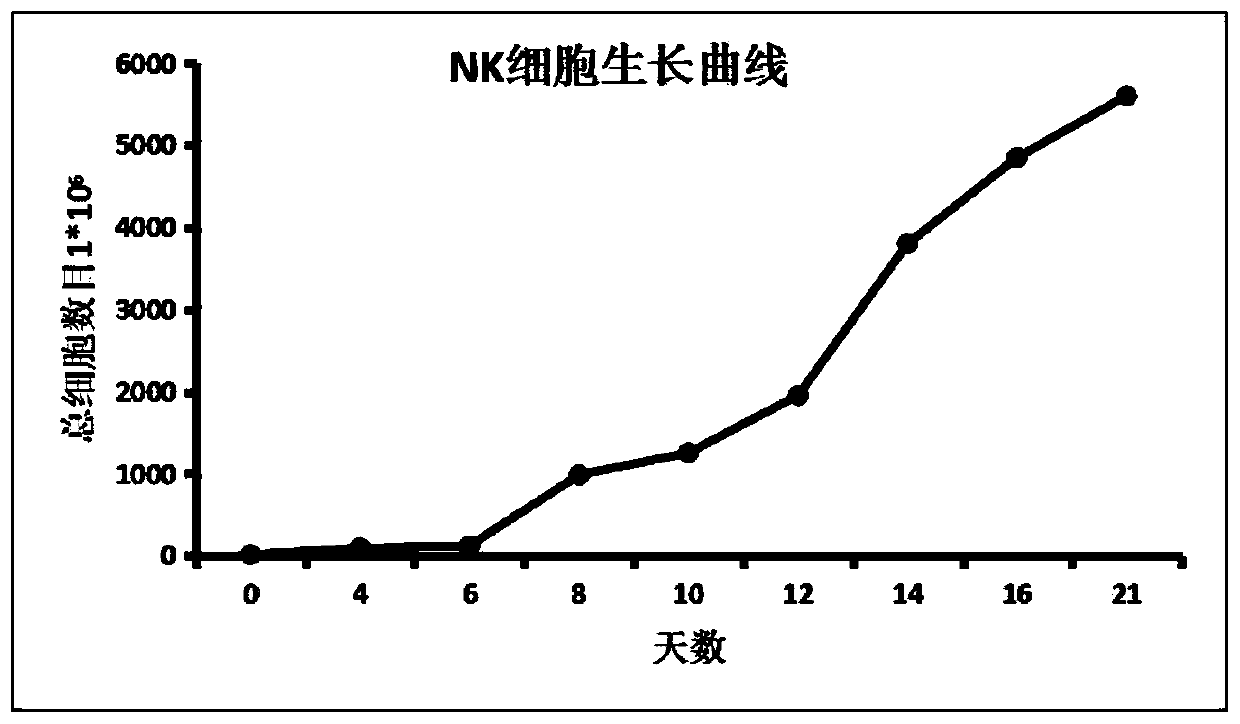
Image
Examples
Embodiment 1
[0038] Example 1 In vitro expansion of NK cells-isolation of monocytes
[0039] 1. Isolation of Monocytes
[0040]1.1 Under the normal working condition of the biological safety cabinet, add Ficoll-Paque Plus lymphocyte separation medium to 50ml sterile centrifuge tubes;
[0041] 1.2 Dilute the patient's peripheral whole blood and DPBS evenly in proportion and slowly inject it into the upper layer of the lymphocyte separation liquid in the centrifuge tube;
[0042] 1.3 20°C, 400×g, centrifuge for 30 minutes;
[0043] 1.4 Use a sterile pipette to draw the plasma layer in the separation tube, put the plasma into a 50ml sterile centrifuge tube, inactivate it in a water bath at 56°C for 30 minutes, centrifuge at 800g×10 minutes, and transfer the upper layer of plasma to a new 50ml sterile centrifuge tube middle;
[0044] 1.5 Pipette the isolated mononuclear cell layer (PBMCs) into a 50ml sterile centrifuge tube;
[0045] 1.6 Add DPBS, mix well, centrifuge at 20°C, 400×g for 10...
Embodiment 2
[0047] Example 2 In vitro expansion of NK cells-CD3 + T cell depletion
[0048] 2.1 According to the counting result of step 1.7, take out 5×10 5 Cells were placed in a 1.5ml EP tube for phenotype detection before sorting. The remaining cells were centrifuged at 20°C and 400×g for 10 minutes, washed once more, and the supernatant was discarded. 1 mM EDTA in DPBS (0.5% HSA, 1 mM EDTA, DPBS) to adjust the cell concentration to 1 × 10 8 cells / ml, transfer to 5ml sterile flow tube, add Easysep at 150μl / ml sample TM Human CD3Positive Selection Cocktail II in Human CD3Positive Selection KitII, mix well, and place at room temperature for 3min;
[0049] 2.2 will Easysep TM Mix RapidSphereTM50100 in Human CD3Positive Selection Kit II, add 90 μl / ml sample to the flow tube in step 2.1, mix well, and place at room temperature for 3 minutes;
[0050] 2.3 Use 0.5% HSA, 1mM EDTA, DPBS to make up the volume of the cell suspension in the 5ml sterile flow tube in step 2.2 to 2.5ml, and ...
Embodiment 3
[0052] Example 3 NK cell expansion in vitro-NK cell culture
[0053] 3.1 According to the counting result in step 2.4, take out 5×10 5 Cells were placed in 1.5ml EP tubes for phenotypic detection after sorting. The remaining cell suspension was supplemented with L500 medium to make up the remaining cell volume to 14ml, mixed well, and centrifuged at 20°C, 400×g for 5min;
[0054] 3.2 Discard the supernatant, centrifuge at 20°C, 400×g for 5 minutes;
[0055] 3.3 Aspirate the supernatant and use SuperCulture containing 10% autologous serum TM L500 (L500) medium to adjust the cell concentration to 1 × 10 6 cells / ml, add IL-2 to make the final concentration 1500IU / ml, and add cytokines (the final concentration of IL-15, Il-12 and IL-21 is 200ng / ml) and OK432 (the final concentration is 2ng / ml) ml), mix well.
[0056] 3.4 Place cells at 37°C, saturated humidity, 5.0% CO 2 cultured in an incubator.
[0057] 3.5 On the 4th day of culture, the cells were blown evenly, and 20 μl ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com