Dual-pH sensitive drug carrier system and preparation method thereof
A carrier system and drug technology, applied in the direction of drug combination, medical formula, medical preparations of non-active ingredients, etc., can solve the problems of releasing drugs and drug carriers are difficult to reach the tumor site, so as to reduce toxic and side effects, and effectively treat cancer cells The effect of internal targeted drug delivery ability, improving drug utilization and encapsulation efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] A preparation method of a double pH-sensitive drug carrier system, specifically comprising the following steps:
[0051] 1. Loading doxorubicin on hollow manganese dioxide and grafting chitosan
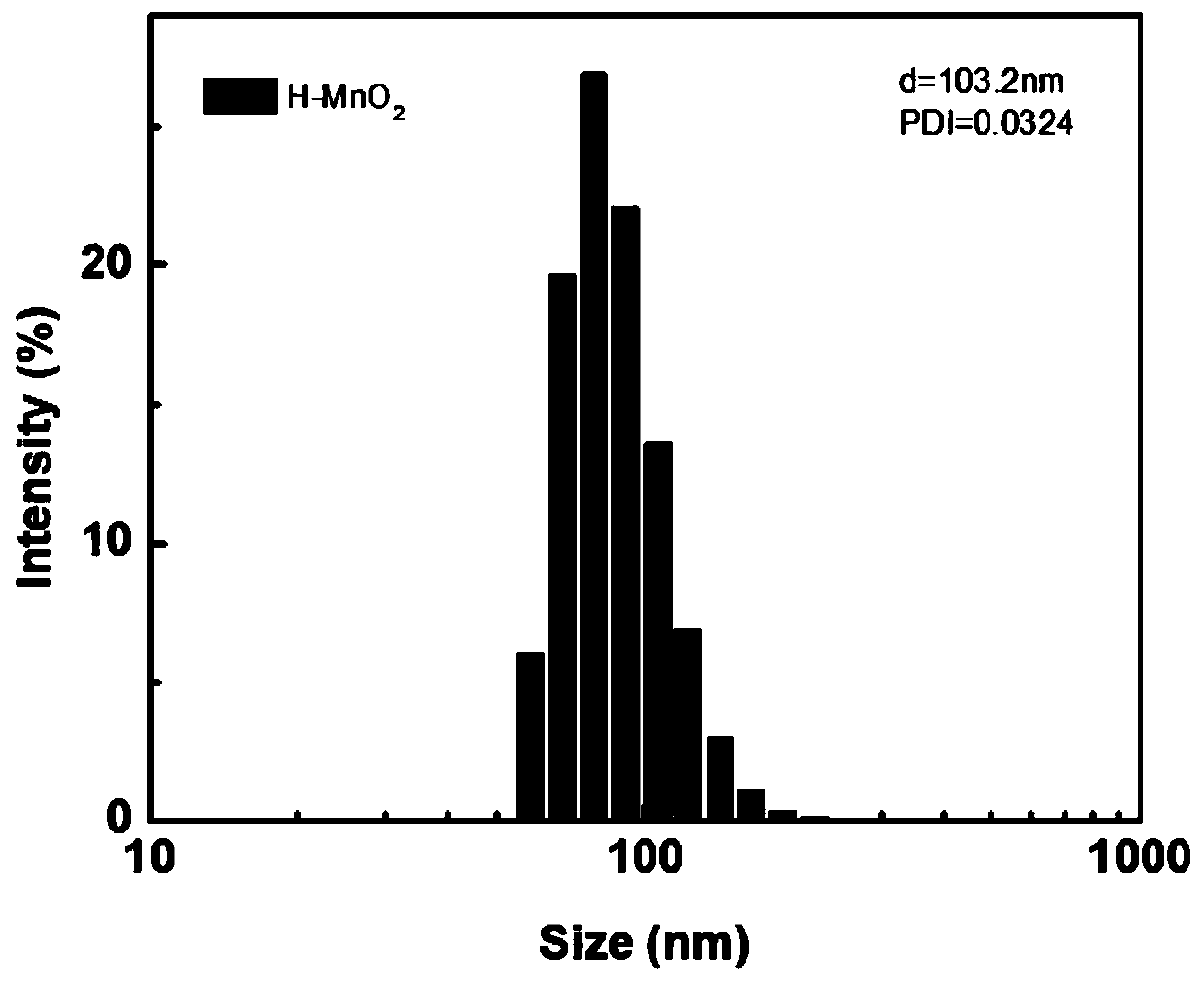
[0052] Disperse 20mg chitosan (CHI) in 120mL deionized water, adjust to 5-6 with acetic acid to obtain solution a; 200mg hollow manganese dioxide H-MnO 2 Disperse in 80mL deionized water, add 100mg DOX, and stir vigorously at 25°C for 24h in the dark to obtain solution b; then add solution a dropwise, react at 25°C for 2.5h, centrifuge, wash and dry to obtain grafted chitosan Sugar and doxorubicin-loaded hollow manganese dioxide (DOX@H-MnO 2 -CHI).
[0053] 2. Modification of monobenzaldehyde-terminated polyethylene glycol
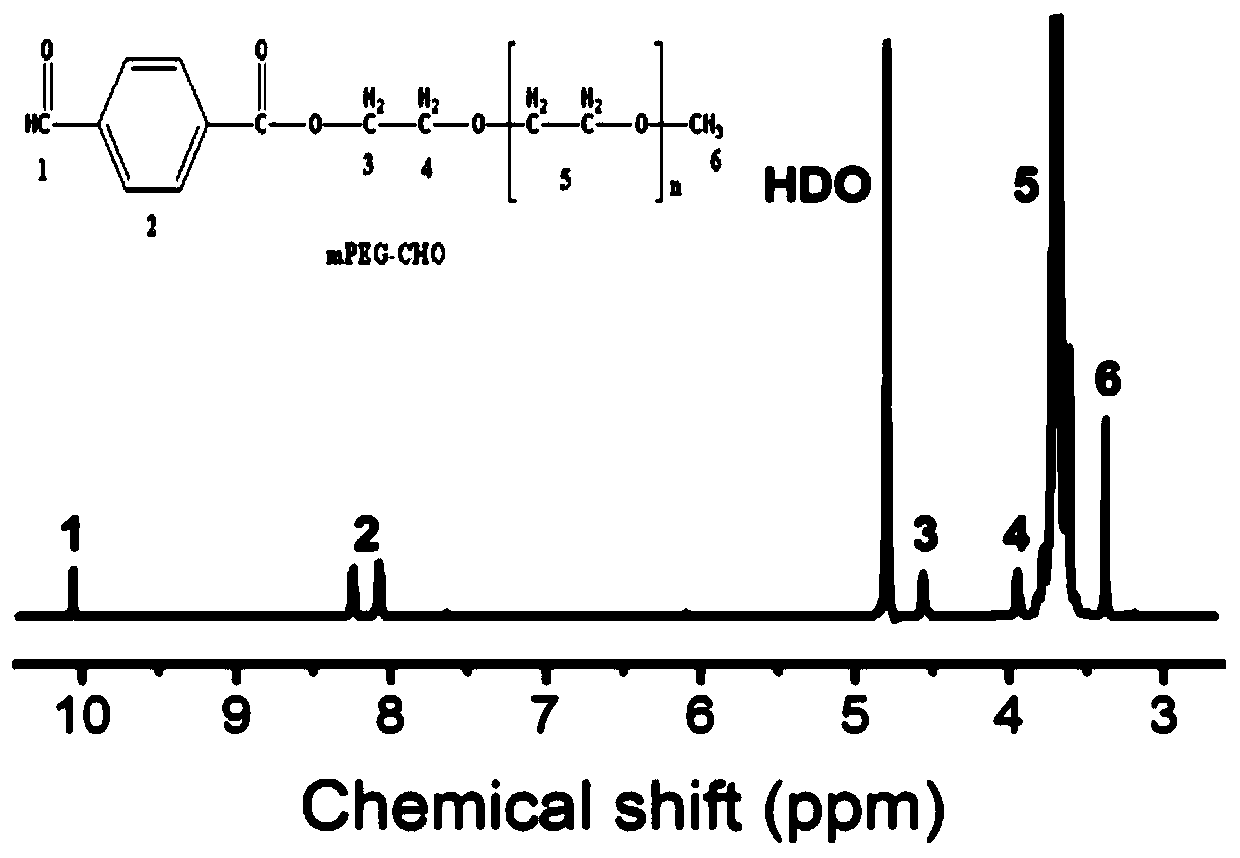
[0054] Accurately weigh 200mg of DOX@H-MnO 2 -CHI, ultrasonically dispersed in 50mL aqueous solution, add one or two drops of dilute hydrochloric acid to adjust the pH to 7-8, then add 200mg of mPEG-CHO to dissolve, continue to react at 25°C for 72h, ce...
Embodiment 2
[0060] A method for preparing a composite nano drug carrier system, specifically comprising the following steps: accurately weighing 200 mg of DOX@H-MnO 2 -CHI, ultrasonically dispersed in 50mL of PBS (pH 7.4) phosphate buffer solution, then added 200mg of mPEG-CHO to dissolve, continued reaction at 25°C for 72h, centrifuged, washed and dried to obtain DOX@H-MnO 2 -CHI-PEG.
Embodiment 3
[0062] A method for preparing a composite nano drug carrier system, specifically comprising the following steps: accurately weighing 200 mg of DOX@H-MnO 2 -CHI, ultrasonically dispersed in 100mL of PBS (pH 7.4) phosphate buffer solution, then added 100mg of mPEG-CHO to dissolve, continued to react at 25°C for 72h, centrifuged, washed and dried to obtain DOX@H-MnO 2 -CHI-PEG.
[0063] The detection method of each intermediate product and final product obtained in Example 2-3 is the same as that of Example 1, and it has been verified that the drug carrier system has been successfully prepared, which has dual pH sensitivity and good targeting, and the drug itself has no large Under the gradual acid activation, it can release the drug well and cause the apoptosis of cancer cells. Therefore, the drug carrier system of the present invention has good dual pH sensitivity and high-efficiency targeted drug delivery ability in cancer cells, which can effectively improve drug utilization...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com