Use of sinomenine or medically acceptable salt thereof for preparing drugs for treatment of ischemic necrosis of femoral head
A technology of ischemic necrosis and sinomenine, which is applied in the direction of medical preparations containing active ingredients, drug combinations, antipyretics, etc., can solve problems such as unpredictable curative effects, achieve no toxic side effects, less trauma, and definite curative effect Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
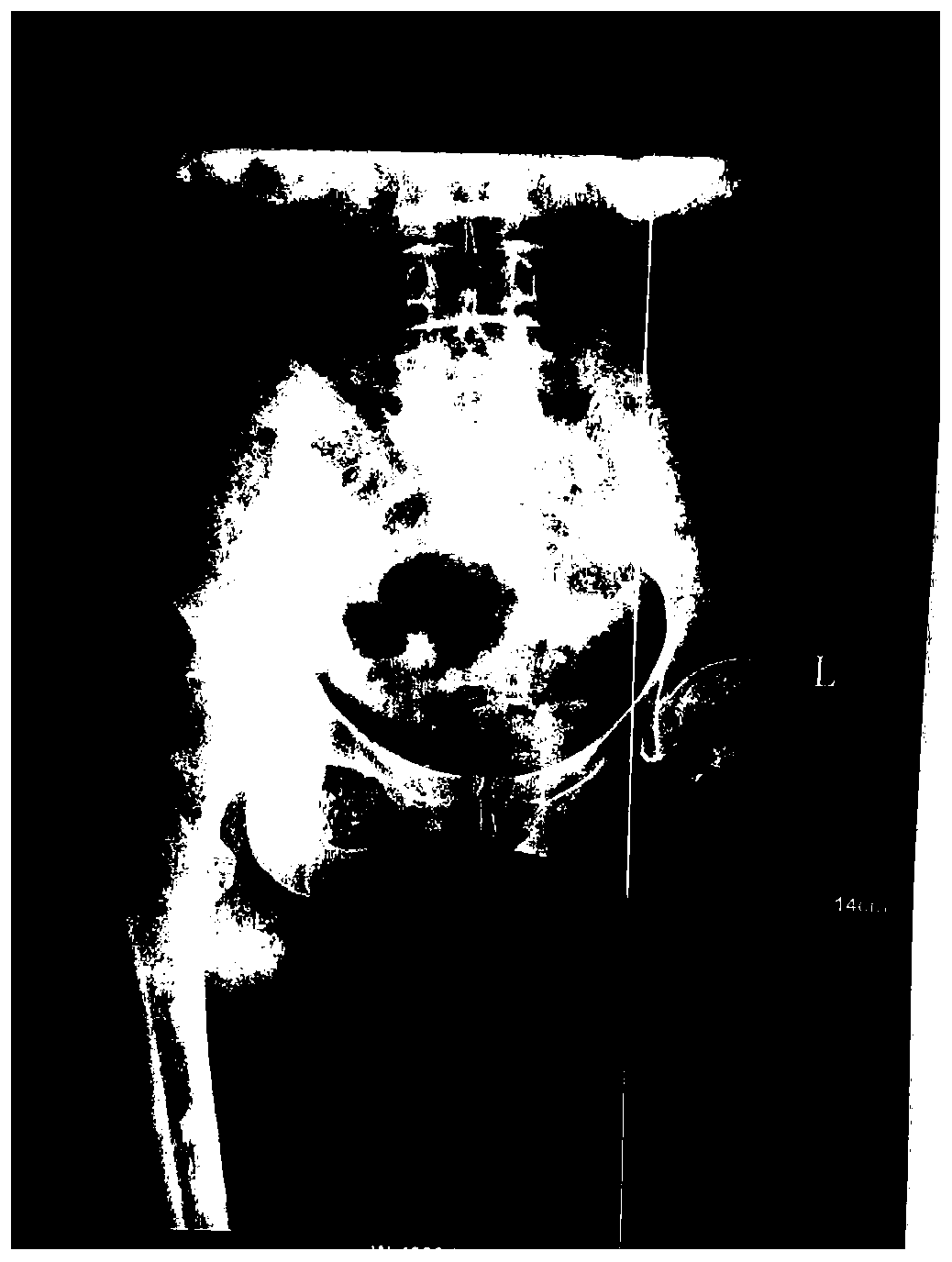
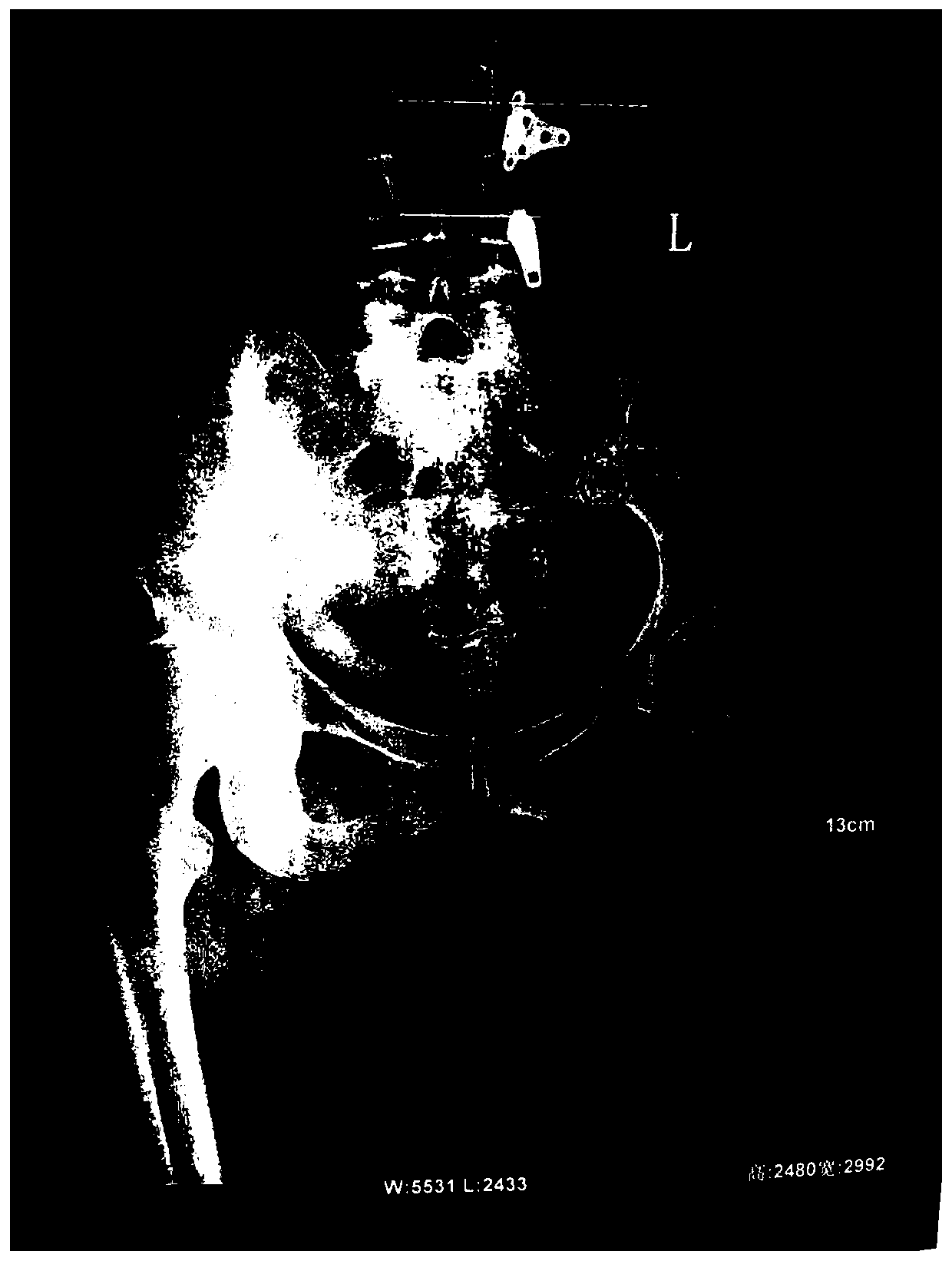
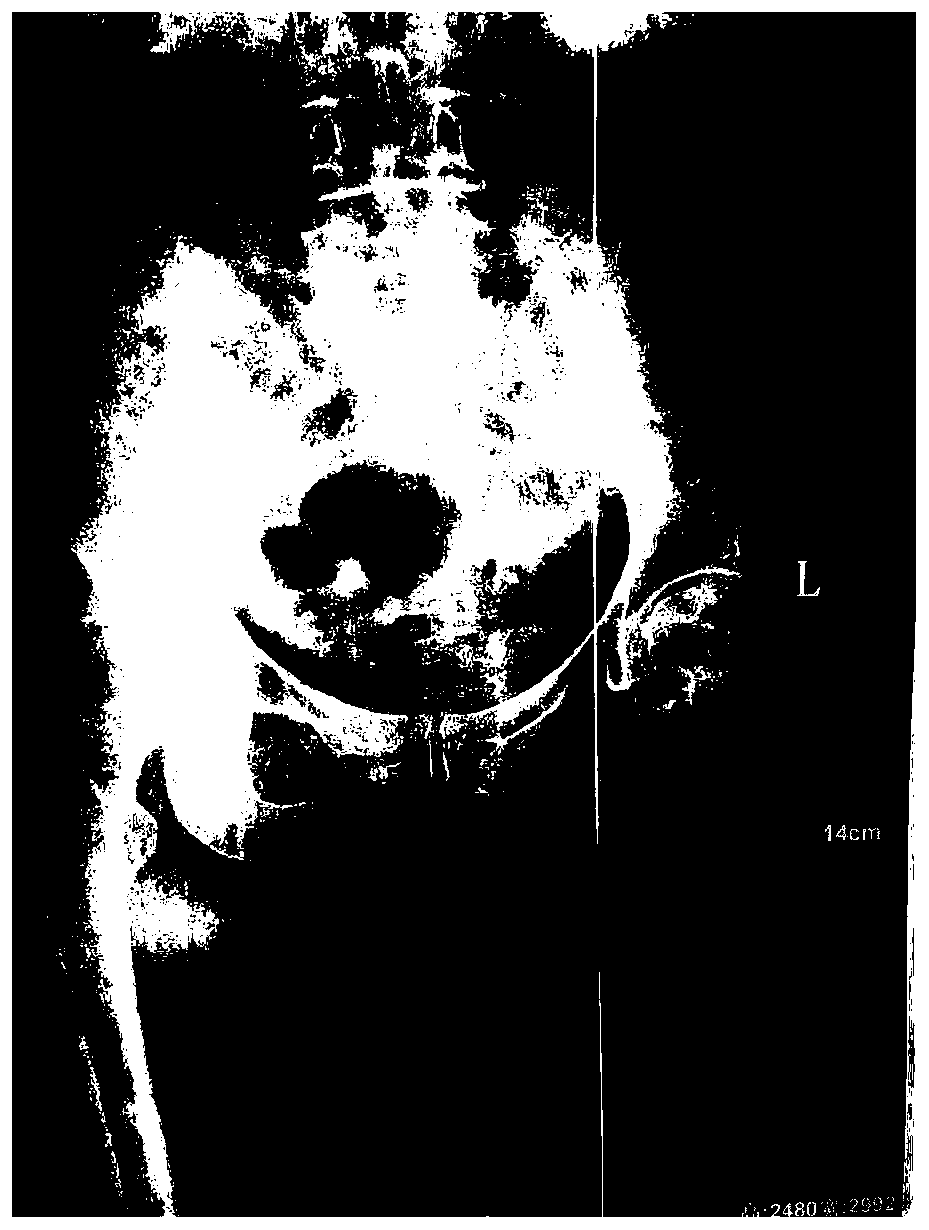
Image
Examples
Embodiment 1
[0038] Take 2ml of 25mg / ml injection. The injection is made of sinomenine hydrochloride as the active ingredient, disodium edetate, sodium bisulfite, and sterile water for injection. The injection is compatible with 2ml of lidocaine hydrochloride. Weekly acupoint injection, once a day, acupoint injection treatment for 7 days as a course of treatment, after a week of rest, continue to the next course of treatment, 6 courses of treatment. On the basis of acupoint injection treatment, the original electroporation combined with 25mg / ml transdermal absorption agent 2ml was used for transdermal administration. The transdermal absorption agent consisted of sinomenine hydrochloride as the active ingredient and edetate disodium Made of sodium hydrogen and sterile water for injection, once a day, 30 minutes each time, transdermal administration for 30 days is a course of treatment, and the medication cycle is 3 courses of treatment.
Embodiment 2
[0040] Take 2ml of 25mg / ml injection, which is made of sinomenine hydrochloride as the active ingredient, disodium edetate, sodium bisulfite, and sterile water for injection, and the injection is compatible with lidocaine hydrochloride 3ml, in the joint cavity and Acupoint injection around the hip, once a day, acupoint injection treatment for 7 days as a course of treatment, after a week of rest, continue to the next course of treatment, 6 courses of treatment. On the basis of acupoint injection treatment, the original electroporation combined with 25mg / ml transdermal absorption agent 4ml was used for transdermal administration. The transdermal absorption agent consisted of sinomenine hydrochloride as the active ingredient and edetate disodium Made of sodium hydrogen and sterile water for injection, once a day, 30 minutes each time, transdermal administration for 30 days is a course of treatment, and the medication cycle is 3 courses of treatment.
Embodiment 3
[0042]Take 3ml of 25mg / ml injection, which is made of sinomenine hydrochloride as the active ingredient, disodium edetate, sodium bisulfite, and sterilized water for injection. The injection is compatible with 2ml of lidocaine hydrochloride. Acupoint injection around the hip, once a day, acupoint injection treatment for 7 days as a course of treatment, after a week of rest, continue to the next course of treatment, 12 courses of treatment. On the basis of acupoint injection treatment, the original electroporation combined with 25mg / ml transdermal absorption agent 2ml was used for transdermal administration. The transdermal absorption agent consisted of sinomenine hydrochloride as the active ingredient and edetate disodium Made of sodium hydrogen and sterile water for injection, once a day, 30 minutes each time, transdermal administration for 30 days is a course of treatment, and the medication cycle is 6 courses of treatment.
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com