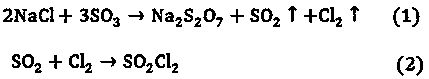Method for co-producing sodium pyrosulfate and sulfuryl chloride
A sodium pyrosulfate and sulfuryl chloride technology, applied in sulfuryl chloride, chemical instruments and methods, alkali metal sulfite/sulfite, etc., can solve high energy consumption, negative corporate benefits, and insufficient value of sodium chloride Cost requirements and other issues, to achieve high quality, reduce environmental hazards, and good economic benefits
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] 1. Take 35.78g of by-product sodium chloride and place it in a four-necked flask, heat it to 420°C in advance, and then slowly introduce sulfur trioxide gas into the four-necked flask. As the reaction progresses, the material first appears to stick to the wall , then this phenomenon disappeared, the surface of the material began to expand, and a small amount of yellow-green gas was released; the reaction was carried out for about 40 minutes, and a large amount of yellow-green gas began to be released uniformly; after the reaction was completed, a total of 178 minutes was carried out, and there was almost no yellow-green gas in the tail gas. 66.86 g of liquid product was obtained, and the material became solid after cooling. The yield was 98.33% based on sodium pyrosulfate, and the content of sodium pyrosulfate was 98.85% as detected by HPLC. According to the material balance calculation, the ratio of the moles of sulfur trioxide fed to the moles of sodium chloride added ...
Embodiment 2
[0029] 1. Take 31.97g of by-product sodium chloride and place it in a four-necked flask, heat it to 500°C in advance, and then slowly introduce sulfur trioxide gas into the four-necked flask. As the reaction progresses, the material first appears to stick to the wall , and then this phenomenon disappeared, the surface of the material began to expand, and a small amount of yellow-green gas was released; the reaction was carried out for about 32 minutes, and a large amount of yellow-green gas began to be released uniformly; after the reaction was completed, a total of 145 minutes was carried out, and there was almost no yellow-green gas in the tail gas. 59.89 g of liquid product was obtained, and the material became solid after cooling. The yield was 98.85% based on sodium pyrosulfate, and the content of sodium pyrosulfate was 99.12% as detected by HPLC. According to the material balance calculation, the ratio of the moles of sulfur trioxide fed to the moles of sodium chloride ad...
Embodiment 3
[0033] 1. Get 44.63g of by-product sodium chloride and place it in a four-necked flask, heat it to 585°C in advance, then slowly feed sulfur trioxide gas with a volume concentration of 90% in the four-necked flask, and as the reaction proceeds, the material The phenomenon of sticking to the wall first appeared, and then this phenomenon disappeared, and the surface of the material began to expand, accompanied by a small amount of yellow-green gas released; the reaction was carried out for about 25 minutes, and a large amount of yellow-green gas began to be released uniformly; the reaction was completed for 122 minutes, and the tail gas was almost no longer emitted. Yellow-green gas was emitted, and a total of 83.73 g of liquid products were obtained. After cooling, the material became solid. The yield was 99.17% based on sodium pyrosulfate. The content of sodium pyrosulfate was 99.28% as detected by HPLC. According to the material balance calculation, the ratio of the moles of s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com