A kind of liposome, its preparation method, liposome assembly and carrier liposome complex
A liposome and CH2 technology, applied in liposome delivery, drug combination, chemical instruments and methods, etc., can solve the problems of non-environmental stimuli responsiveness, poor repeatability, and low drug concentration, and achieve reliable Control the film forming temperature, broad application prospects, promote the effect of cell internalization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
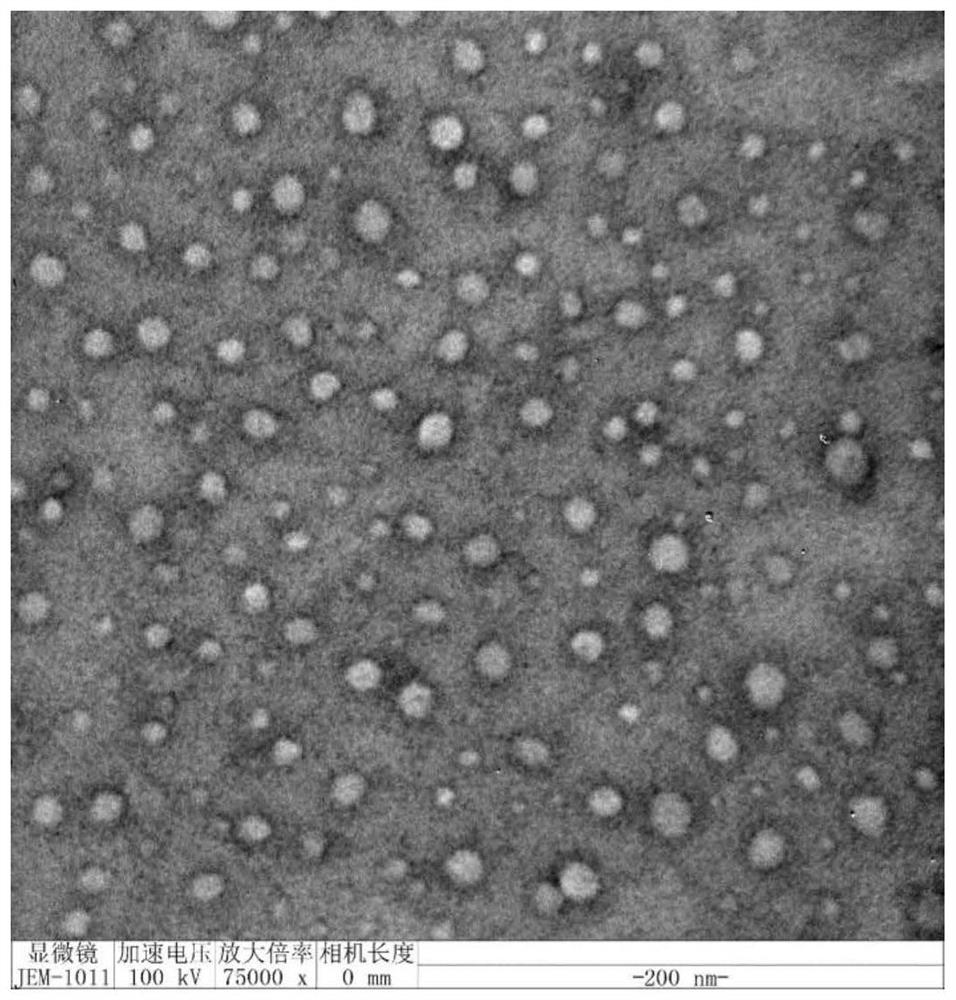
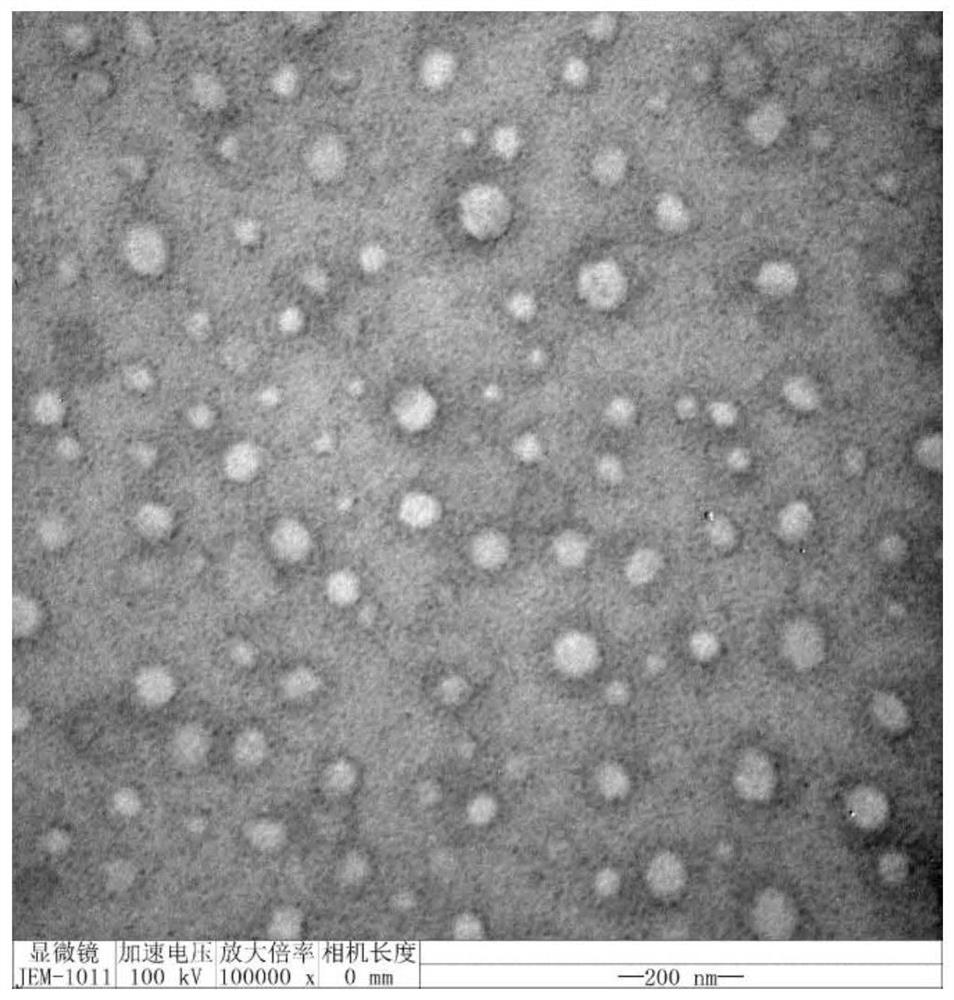
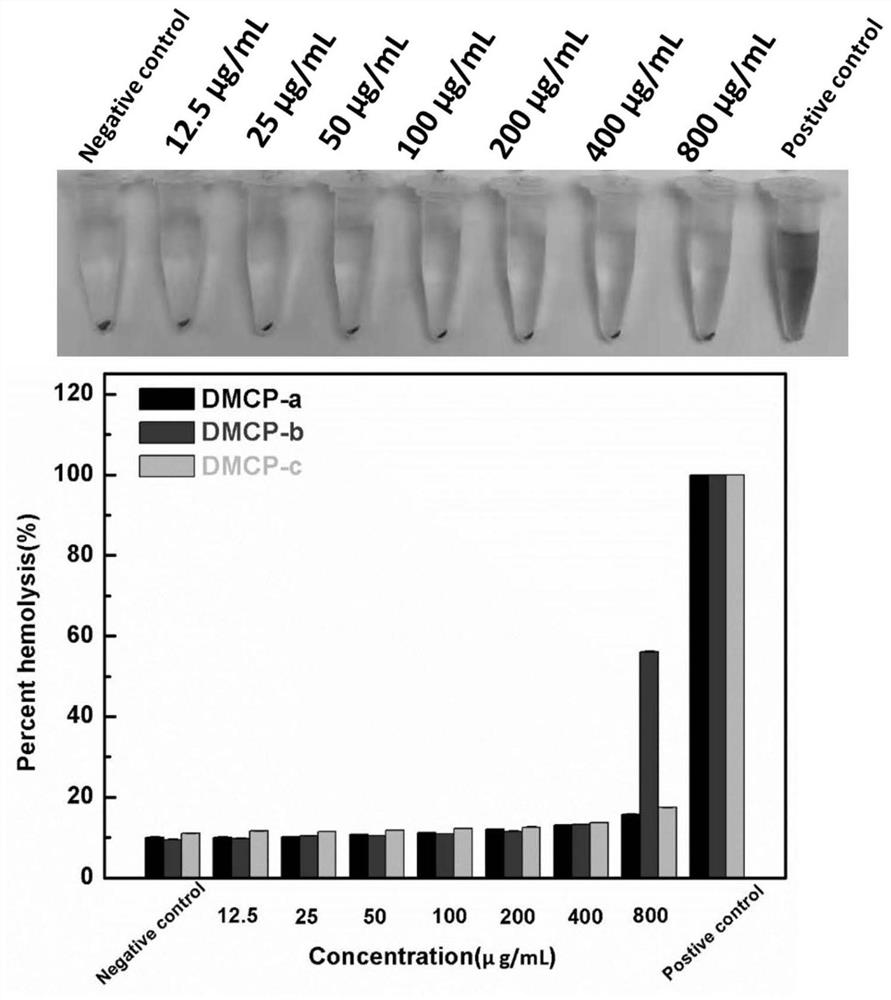
Image
Examples
preparation example Construction
[0126] According to the present invention, the first preparation method of the liposome comprises the following steps:
[0127] a) compound A 1 -COOH, Compound A 2 -COOH is reacted with 1,2-propanediol derivative X-1 to obtain compound Y-1;
[0128] b) reacting the phosphinocyclopentane compound X-2 with the dimethylamine compound X-3 to obtain the compound Y-2;
[0129] c) reacting the compound Y-1 with the compound Y-2 to obtain liposomes represented by formula (I);
[0130] The steps a) and b) have no order limitation;
[0131]
[0132]
[0133] in,
[0134] A 1 and A 2 Independently selected from hydrocarbyl C x h 2x+y ; x is an integer from 5 to 35, and y is 1, -1, -3, -5, -7, -9 or -11;
[0135] no 1 , n 2 , L 1 and L 2 The options are as follows:
[0136] no 1 =0,n 2 =0,L 1 and L 2 Linked to form -(CH 2 ) n3 -, n 3 1 to 4. Among them, L 1 and L 2 There are no special restrictions on the type of the two reacting to form -(CH 2 ) n3 - can b...
Embodiment 1~13
[0205] The preparation of embodiment 1~13 compound Y-1
[0206]
Embodiment 1~3
[0208] Add 56g of hexadecanoic acid (0.22mol), 12g of N,N-dimethylamino-1,2-propanediol (0.1mol) into a 500mL reaction flask, add 300mL of dichloromethane, stir well and add 27.8g of N, N'-diisopropylcarbodiimide (DIC) (0.22mol) and 0.2g 4-dimethylaminopyridine (DMAP), after fully stirring and reacting at room temperature for 24h, filtered, and the solid obtained after the filtrate rotary distillation was further recombined Through crystallization and purification, 54 g of the product was obtained with a yield of 96.8%.
[0209] According to the above-mentioned preparation process, the difference is that the 56g hexadecanoic acid in the above-mentioned preparation process is replaced with 62g oleic acid and 61g octadecatrienoic acid (both 0.22mol) respectively. The yield and product structure of the obtained product are shown in Table 1.
[0210] The productive rate and structure of table 1 embodiment 1~3
[0211]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com