A tertiary amine nitrogen-containing polysaccharide derivative with controllable switching of hydrophilicity/hydrophobicity and its preparation method and application
A hydrophobic, tertiary amine-type technology, applied in the direction of flocculation/sedimentation water/sewage treatment, etc., to achieve the effect of improving adsorption bridging ability, energy saving, emission reduction and environmental protection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
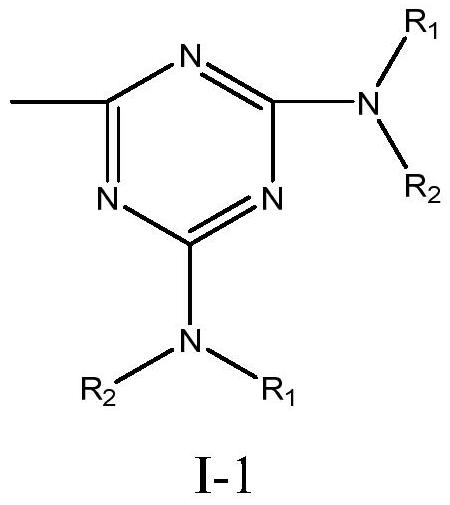
[0051] Add 0.1mol of 1# compound, 0.05mol of starch (calculated as polysaccharide unit), 0.03mol of ethoxyglycidyl ether, and 120g of DMSO into the reactor, raise the temperature to 100°C, and react for 10h under stirring. After washing with methanol, filtering, drying and pulverizing, a tertiary amine nitrogen-containing starch derivative with controllable switching of hydrophilicity / hydrophobicity is obtained.
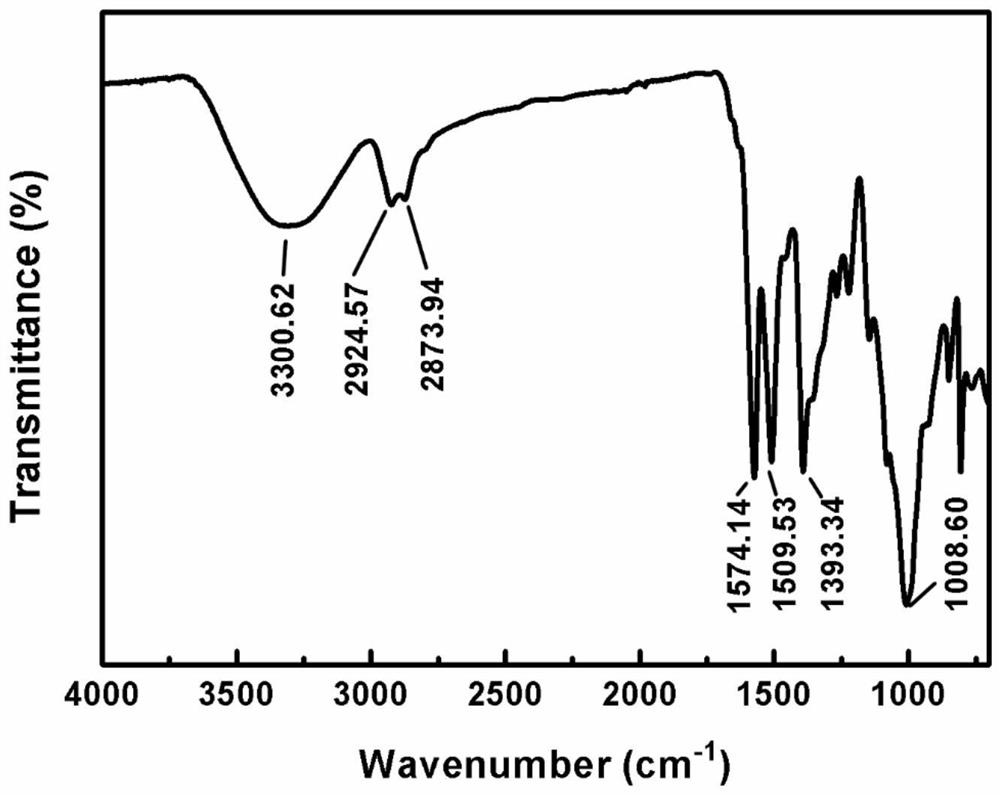
[0052] The above product was subjected to dialysis treatment to remove unreacted reagents, and after drying, infrared analysis was performed. attached figure 1 In the infrared spectrogram of the product, the characteristic peak of the triazine ring (1586,1501cm -1 ), methyl C-H characteristic peak (2922cm -1 ), C-O-C characteristic peak (1006cm -1 ), indicating that compound 1#, ethoxyglycidyl ether, reacted chemically with starch, and obtained tertiary amine nitrogen-containing starch derivatives with controllable switching of hydrophilicity / hydrophobicity.
[0...
Embodiment 2~12
[0057] The method is the same as in Example 1, that is, using chitosan, chitin, cellulose, hemicellulose, guar gum, xylan, glucomannan, barley β-glucan, β-1,3 glucan , glycogen, and cyclodextrin instead of starch, and react with 1# compound and ethoxyglycidyl ether in Example 1 to obtain tertiary amine nitrogen-containing polysaccharide derivatives with controllable conversion of hydrophilicity / hydrophobicity.
Embodiment 13
[0059] Add 0.1 mol of 1# compound, 0.05 mol of starch (calculated as polysaccharide unit), 0.03 mol of benzyl chloride, and 120 g of DMSO into the reactor, raise the temperature to 120°C, and react for 8 hours under stirring. Solids are precipitated in water, washed with anhydrous methanol, and filtered , drying and pulverizing to obtain tertiary amine nitrogen-containing starch derivatives with controllable conversion of hydrophilicity / hydrophobicity.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com