Actinic-ray-curable ink and production method for printed matter
An active energy ray, curable technology, applied in the direction of replication/marking method, ink, printing, etc., can solve the problems of reduced adhesion performance, no discovery, etc., to achieve the effect of excellent adhesion performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1~6 and comparative example 1~4
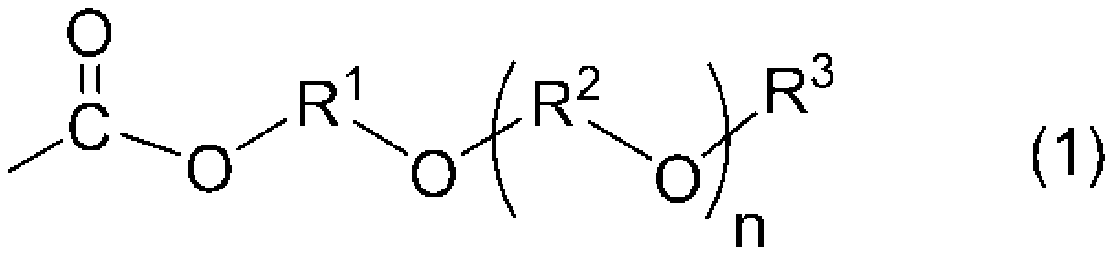
[0136] According to the compounding ratio described in Table 1 and Table 2, the active energy ray curable ink which can be used for inkjet printing was manufactured. Specifically, MIRAMER M3130 (ethylene oxide (EO)-modified trimethylolpropane triacrylate manufactured by MIWON Corporation), MIRAMER M240 (ethylene oxide (EO)-modified bisphenol A diacrylate), V-190 (ethoxyethoxyethyl acrylate manufactured by Osaka Organic Chemical Industry Co., Ltd.), IBXA (isobornyl acrylate manufactured by Osaka Organic Chemical Industry Co., Ltd.), V-CAP (manufactured by ISP Co., Ltd. N-vinyl-2-caprolactam), LIGHT ACRYLATE POA (phenoxyethyl acrylate manufactured by Kyoeisha Chemical Co., Ltd.), tetrahydrofurfuryl acrylate composition containing a trace amount of tetrahydrofurfuryl alcohol, KF-54 (Shin-Etsu Chemical Co., Ltd. Co., Ltd. polysiloxane), stirred and mixed, then added Irgacure 819 (bis(2,4,6-trimethylbenzoyl)-phenylphosphine oxide manufactured by BASF Corporation), Irgacure.TPO (man...
Embodiment 7
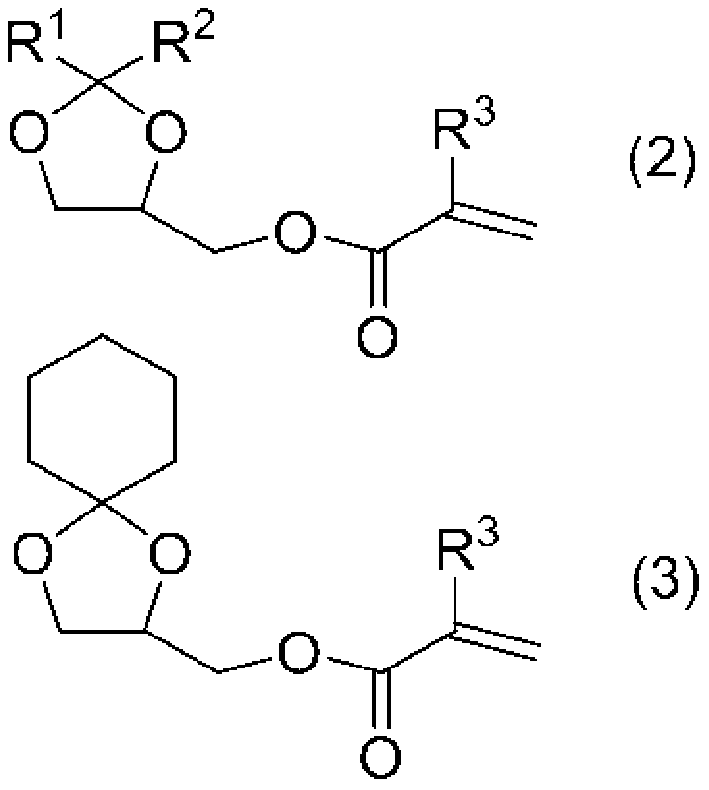
[0178] According to the compounding ratio recorded in Example 7 of Table 3, MIRAMER M3130 (ethylene oxide (EO) modified trimethylolpropane triacrylate manufactured by MIWON Corporation), MIRAMER M240 (ethylene oxide (EO) modified by MIWON Corporation, alkane (EO) modified bisphenol A diacrylate), MEDOL-10 (manufactured by Osaka Organic Chemical Industry Co., Ltd., acrylic acid (2-methyl-2-ethyl-1,3-dioxolan-4-yl ) methyl ester), IBXA (isobornyl acrylate manufactured by Osaka Organic Chemical Industry Co., Ltd.), V-CAP (N-vinyl-2-caprolactam manufactured by ISP Corporation), LIGHT ACRYLATE POA (acrylic benzene manufactured by Kyoeisha Chemical Co., Ltd.) Oxyethyl ester), KF-54 (polysiloxane manufactured by Shin-Etsu Chemical Co., Ltd.), and stirred to mix, then add Irgacure 819 (bis(2,4,6-trimethylbenzoyl) manufactured by BASF -Phenylphosphine oxide), Irgacure.TPO (2,4,6-trimethylbenzoyl-diphenyl-phosphine oxide manufactured by BASF Corporation), Kayacure DETX-S (diethylthiophe...
Embodiment 8
[0181] The amount of MEDOL-10 (manufactured by Osaka Organic Chemical Industry Co., Ltd., (2-methyl-2-ethyl-1,3-dioxolan-4-yl) methyl acrylate) was changed from 20 parts by mass to 27 parts by mass, the amount of POA (phenoxyethyl acrylate produced by Kyoeisha Chemical Co., Ltd.) was changed from 16.3 parts by mass to 9.3 parts by mass, except that the active energy was obtained by the same method as in Example 7 Radiation curable ink (C8).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com