6-oxadiazole/thiadiazole chitooligosaccharide derivative as well as preparation and application thereof
A technology of thiadiazole chitosan oligosaccharides and derivatives, which is applied in the field of novel 6-oxa/thiadiazole chitosan oligosaccharides derivatives and their preparation and application, can solve the problems that the activity cannot reach the level of direct application, etc. Achieve the effects of improving biocompatibility, improving inhibitory activity, and expanding application fields
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0040] The preparation method of the derivative of the present invention: dissolving chitosan oligosaccharide in 13.5% lye, stirring at 50-70°C for 1-2h, then dissolving chloroacetyl in isopropanol equivalent to one-fifth of the volume of lye , and then added to chitosan oligosaccharide lye, and continued to react for 4-6h. After the reaction, the pH of the reaction solution was adjusted to be neutral, dialyzed, and freeze-dried to obtain 6-carboxymethyl chitosan oligosaccharide.
[0041] Disperse 6-carboxymethylchitooligosaccharide in a mixture of dichloromethane and pyridine (V:V=3:1), add DCC and DMAP and stir at room temperature for 18-24h, then add methanol and continue the reaction at room temperature 4-6h. After the reaction is finished, filter, wash the filter cake with ethanol and DMF, and dry the filter cake at 50-60° C. to obtain 6-methyl acetate chitosan oligosaccharide.
[0042] Dissolve 6-methyl acetate chitooligosaccharide in 50% ethanol, then react with hydraz...
Embodiment 1
[0045] The preparation of embodiment 1 6-oxadiazole chitosan oligosaccharide:
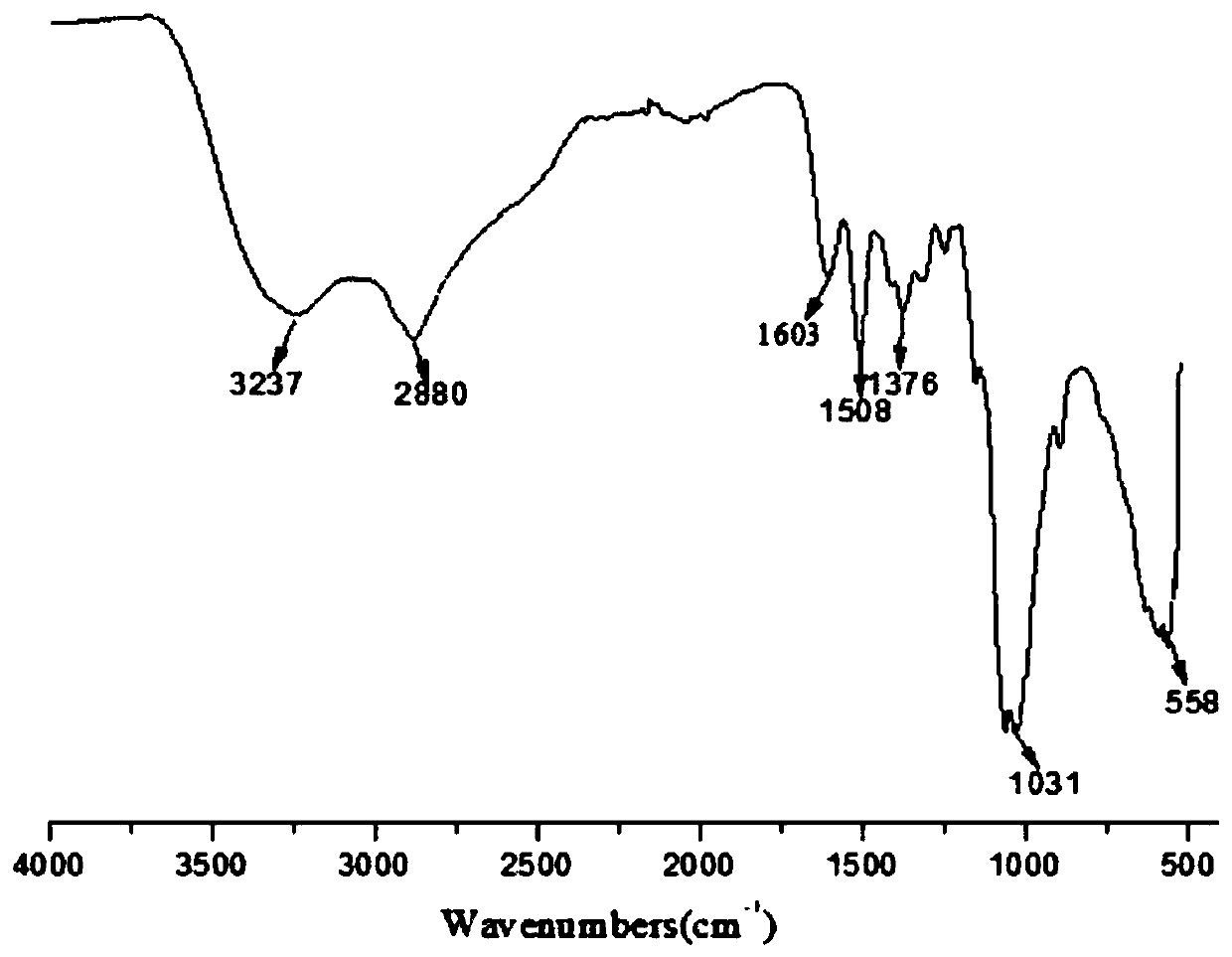
[0046] Weigh 5g chitosan oligosaccharide and dissolve in 50ml lye (6.75gNaOH / 50mlH 2 (0), stirred at 50° C. for 1 h, weighed 5.4 g of chloroacetic acid and dissolved it in 10 ml of isopropanol, then added the solution dropwise to the above-mentioned chitosan oligosaccharide solution within 30 min, and continued the reaction for 4 h. After the reaction finished, adjust the reaction solution to neutrality with 1M hydrochloric acid, then use the dialysis bag of 1000 molecular weight for dialysis, freeze-drying subsequently, obtain 4.2g yellow solid 6-carboxymethyl chitosan oligosaccharide (see figure 2 ).
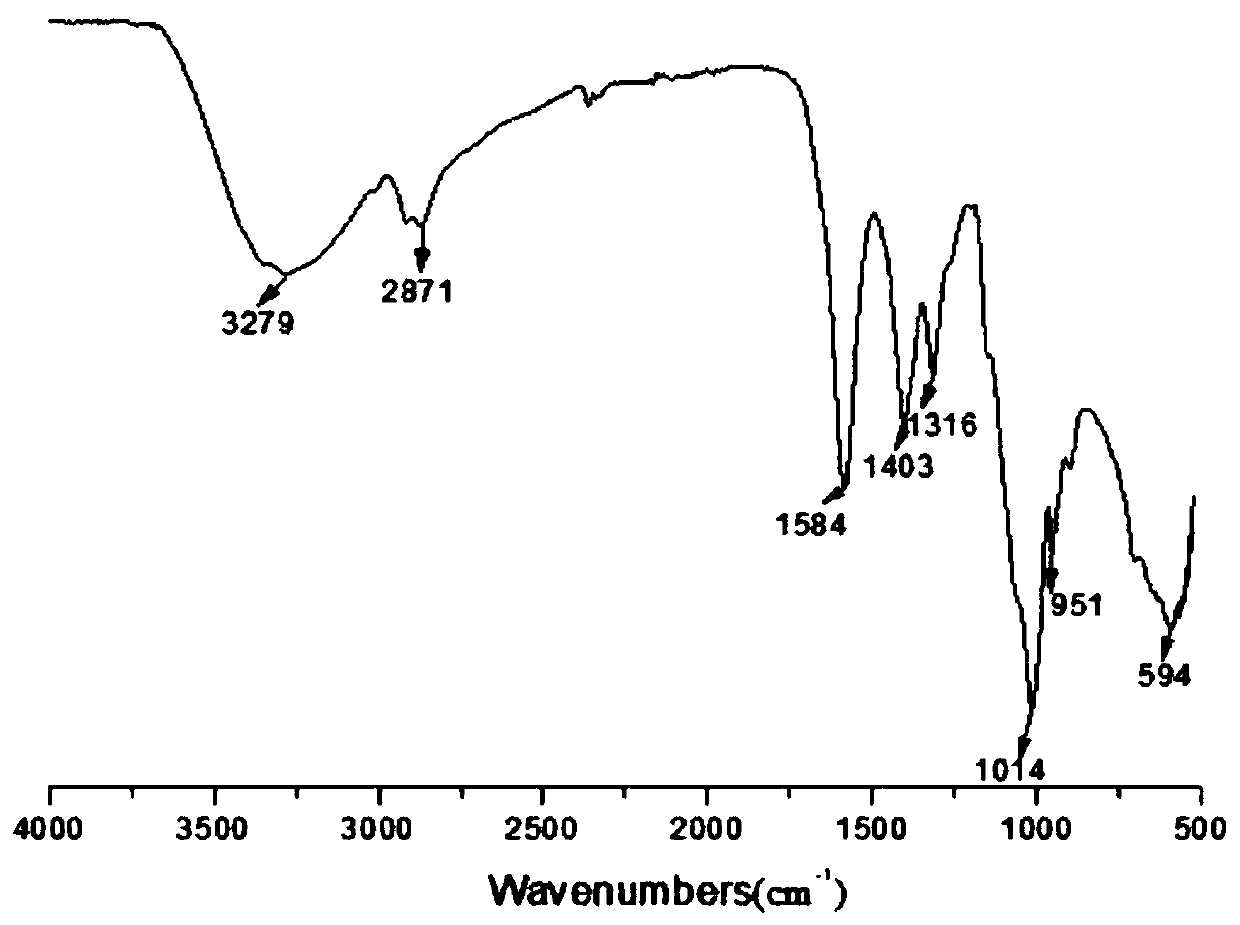
[0047] Weigh 4g 6-carboxymethylchitooligosaccharide, 2g dicyclohexylcarbodiimide and 0.2g 4-diaminopyridine and disperse in 40ml organic solvent (methylene chloride / pyridine=3:1), stir at room temperature After 6 hours, 4ml of methanol was slowly added dropwise to the reaction system, and the re...
Embodiment 2
[0051] The preparation of embodiment 2 6-thiadiazole chitosan oligosaccharides:
[0052] Weigh 5g chitosan oligosaccharide and dissolve in 50ml lye (6.75gNaOH / 50mlH 2 (0), stirred at 50° C. for 1 h, weighed 5.4 g of chloroacetic acid and dissolved it in 10 ml of isopropanol, then added the solution dropwise to the above-mentioned chitosan oligosaccharide solution within 30 min, and continued the reaction for 4 h. After the reaction, the reaction solution was adjusted to neutrality with 1M hydrochloric acid, then dialyzed with a 1000 molecular weight dialysis bag, and then freeze-dried to obtain 4.2 g of yellow solid 6-carboxymethyl chitosan oligosaccharide.
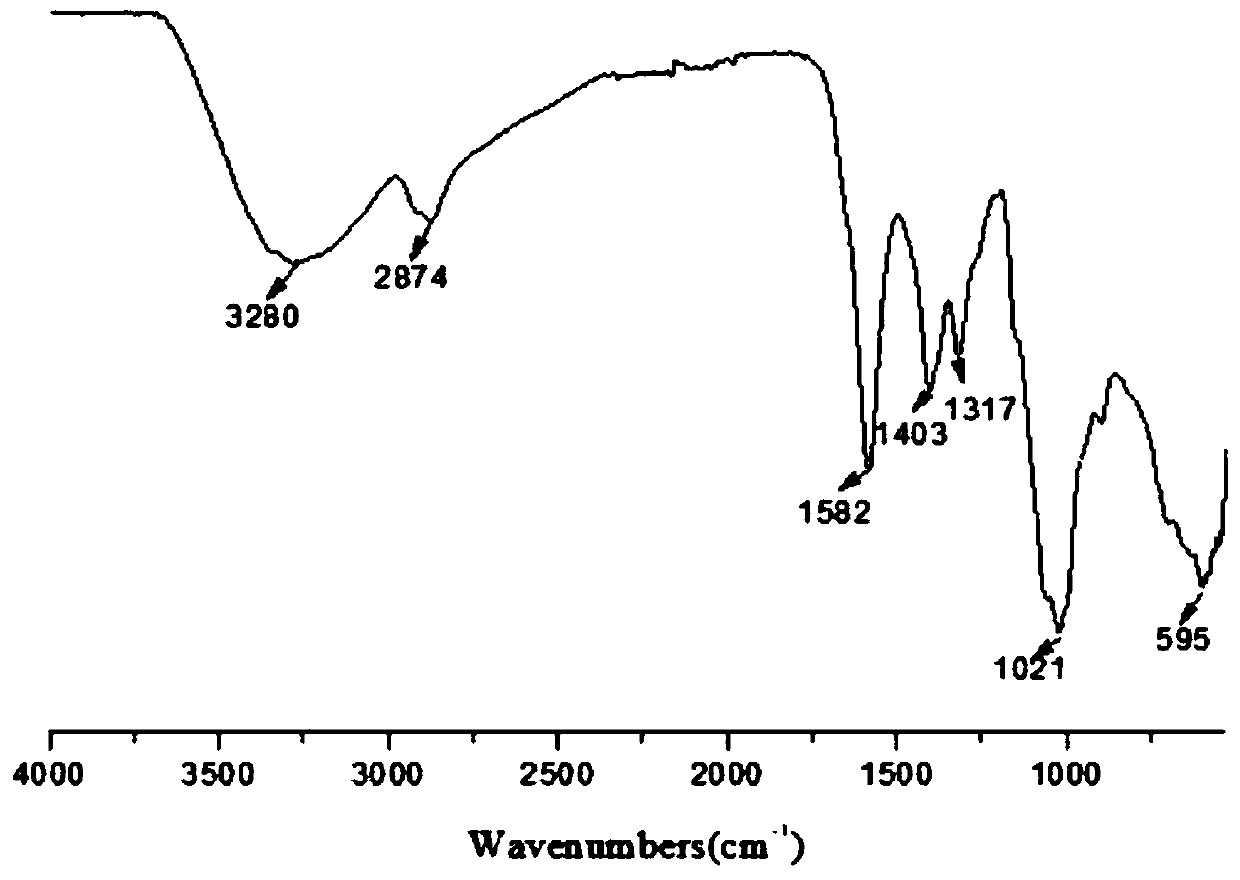
[0053] Weigh 4g 6-carboxymethylchitooligosaccharide, 2g dicyclohexylcarbodiimide and 0.2g 4-diaminopyridine and disperse in 40ml organic solvent (methylene chloride / pyridine=3:1), stir at room temperature After 6 hours, 4ml of methanol was slowly added dropwise to the reaction system, and the reaction was continued for 1...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com