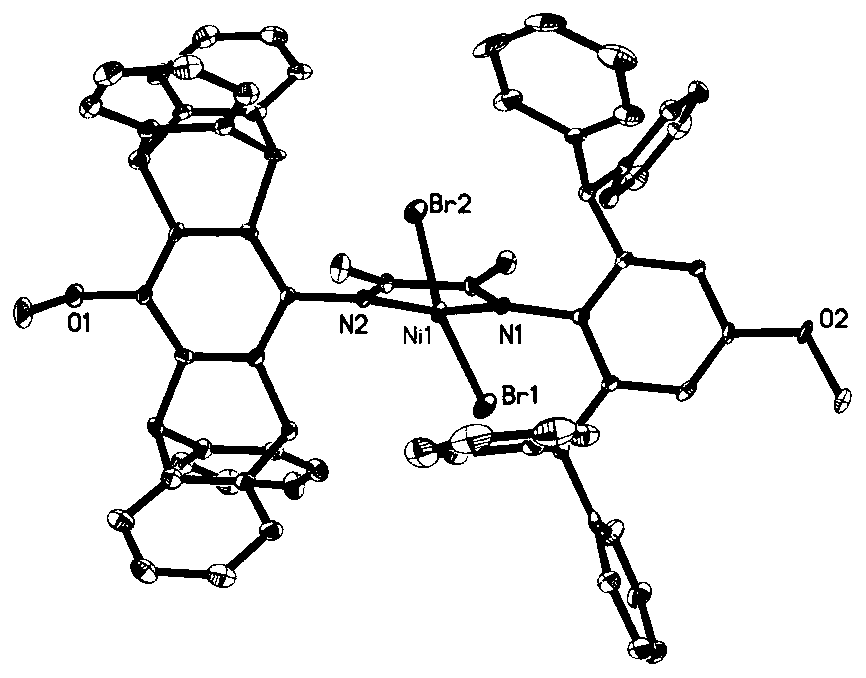
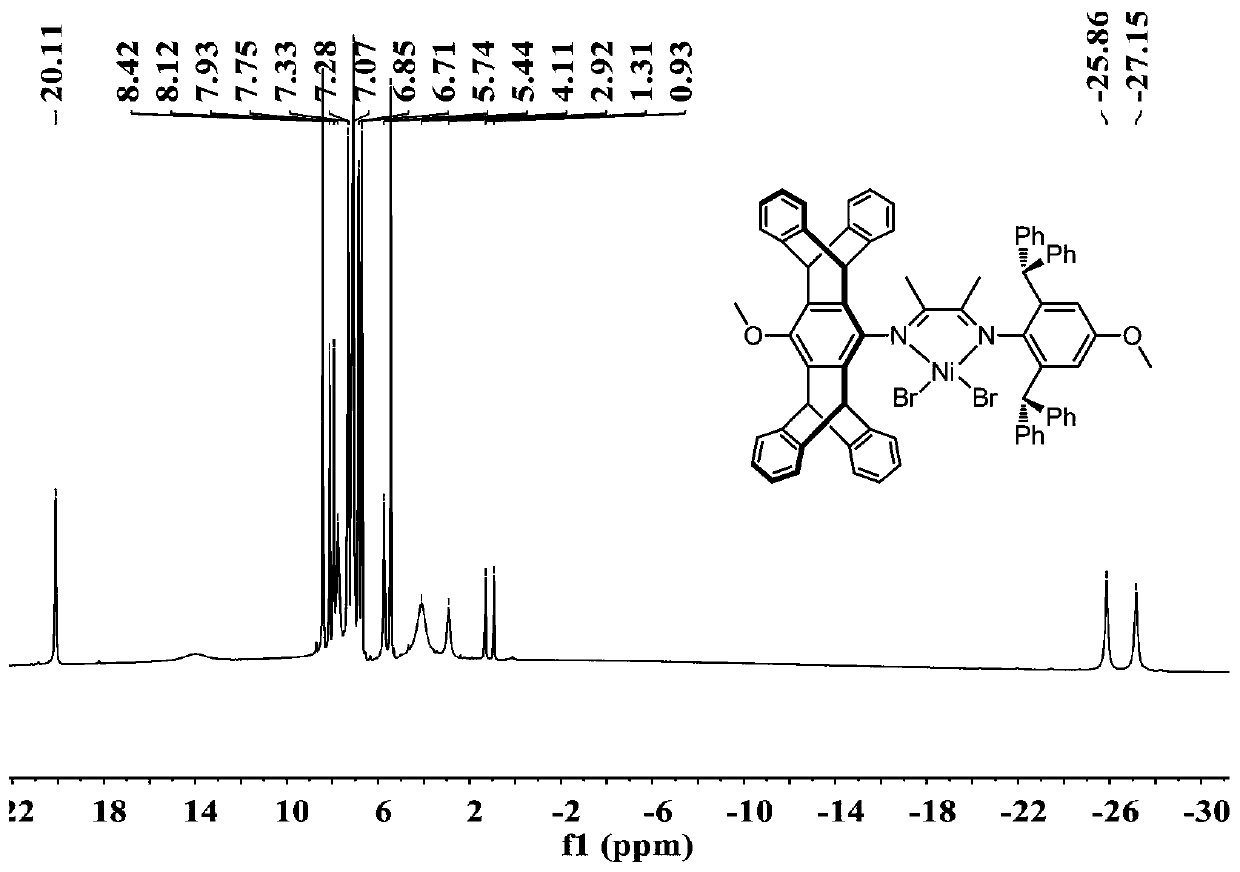
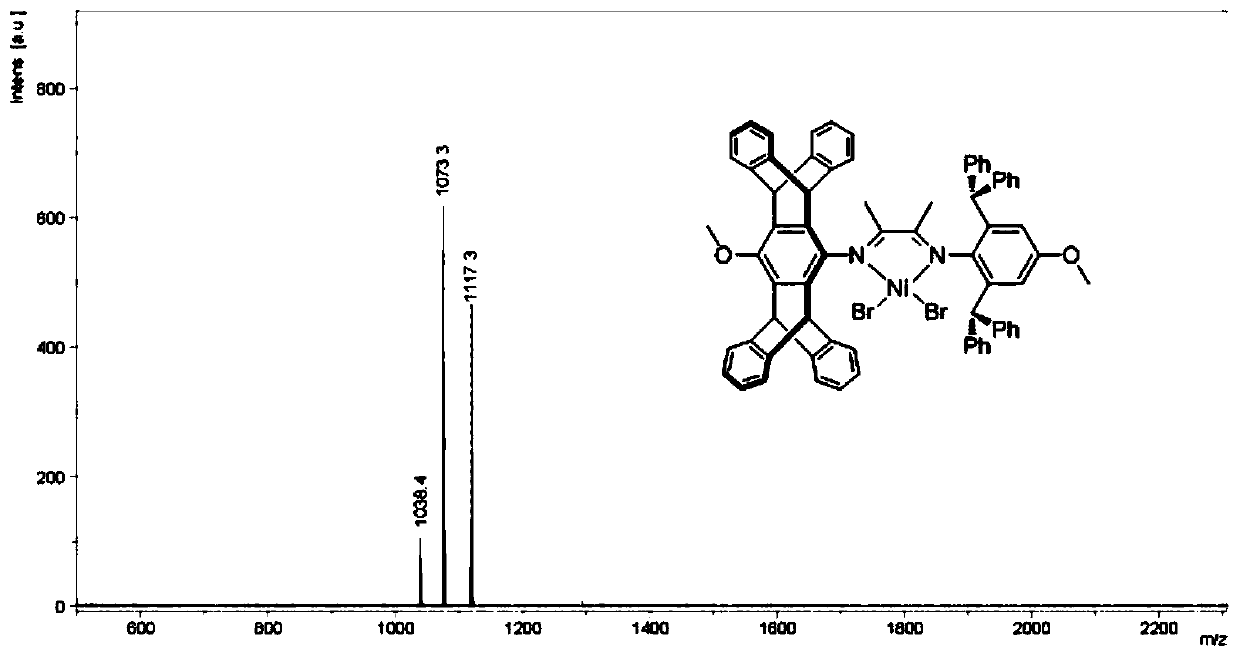
Asymmetric alpha-diimine nickel catalysts as well as preparation method and application thereof
A nickel diimide, asymmetric technology, applied in the field of asymmetric α-diimine nickel catalyst and its preparation, can solve the problem of low degree of polymer branching and the like, achieve wide processing methods, reduce processing costs, and facilitate processing Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] The preparation method of asymmetric α-diimine nickel catalyst of the present invention, the steps are as follows:
[0057] Step 1, dissolving the aniline represented by formula (a) and the diketone represented by formula (b) in an organic solvent according to the ratio of substances being 1:N, adding a catalyst amount of catalyst, and stirring at 25-80° C. for more than 6 h, Cool to room temperature, evaporate the organic solvent until a yellow solid appears, precipitate the yellow solid, filter, wash, and vacuum-dry to obtain the monoimine product represented by formula (d), and the yield can reach more than 90%;
[0058] Among them, N≥1, the larger N is, the shorter the reaction time is, and the product conversion can be improved;
[0059] Step 2, dissolving the monoimine product prepared in step 1 and the aniline represented by formula (c) in an organic solvent according to the ratio of the substances of 1:1, adding a catalyst amount of catalyst, stirring and reacti...
Embodiment 1
[0075] Step 1, 2,6-diphenylmethyl-4-hydroxyaniline (6.00g, 13.59mmol), 2,3-butanedione (5.85g, 67.95mmol) and p-toluenesulfonic acid monohydrate (20mg ) was dissolved in toluene (200mL), stirred at 80°C and maintained for 24h, cooled to room temperature, and evaporated toluene by rotary evaporation until a yellow solid appeared, and an excess of methanol was added to precipitate the product, the yellow solid was separated by filtration, washed three times with methanol, and Dry to obtain a yellow solid product (5.23g, 75.5% yield), namely 2-(2,6-benzhydryl-4-hydroxyphenylimino) butanone, the structural formula is as follows:
[0076]
[0077] Step 2, 2-(2,6-diphenylmethyl-4-hydroxyphenylimino) butanone (2.00g, 3.92mmol), 4-hydroxypentadecene aniline (1.81g) obtained in step 1 , 3.92mmol) and p-toluenesulfonic acid monohydrate (20mg) were dissolved in toluene (250mL), heated to reflux at 120°C for 3 days, cooled to room temperature, and evaporated methanol until a yellow sol...
Embodiment 2~19
[0084] Steps 1 to 4 are the same as in Example 1, except that the R of the asymmetric α-diimine nickel catalyst is 1 =CH 3 , R 2 =H,R 3 =OCH 3 ,R 4 and R 5 As shown in Table 1. The properties of the obtained polyethylene were tested, and the results are shown in Table 1.
[0085] (R 4 , R 5 ) The structure of the asymmetric α-diimine nickel catalyst and the performance of the catalyzed polyethylene
[0086]
[0087]
[0088] In Table 1, activity: take 10 6 gmol -1 h -1 as the unit. m w is the weight average molecular weight, M w / M n is the polymer dispersibility index, determined by GPC in 1,2,4-trichlorobenzene at 150°C, relative to polystyrene standards. Branching degree = the number of branches per 1000 carbons, determined by proton nuclear magnetic resonance spectroscopy. All data in Table 1 are based on at least two parallel experiments (unless otherwise stated).
[0089] As can be seen from Table 1, when controlling the catalyst substituent R 1 ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com