Heat-resistant serum 4 type fowl adenovirus genetic engineering vaccine candidate strain and construction method thereof
A genetically engineered vaccine and avian adenovirus technology, applied in the field of genetically engineered vaccines, can solve the problems of loss of activity, loss of the ability to infect the host, and low tolerance.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0043] Embodiment 1: Serum type 4 poultry adenovirus genetic engineering vaccine strain construction
[0044] 1. Digestion of plasmid rVHR09-P
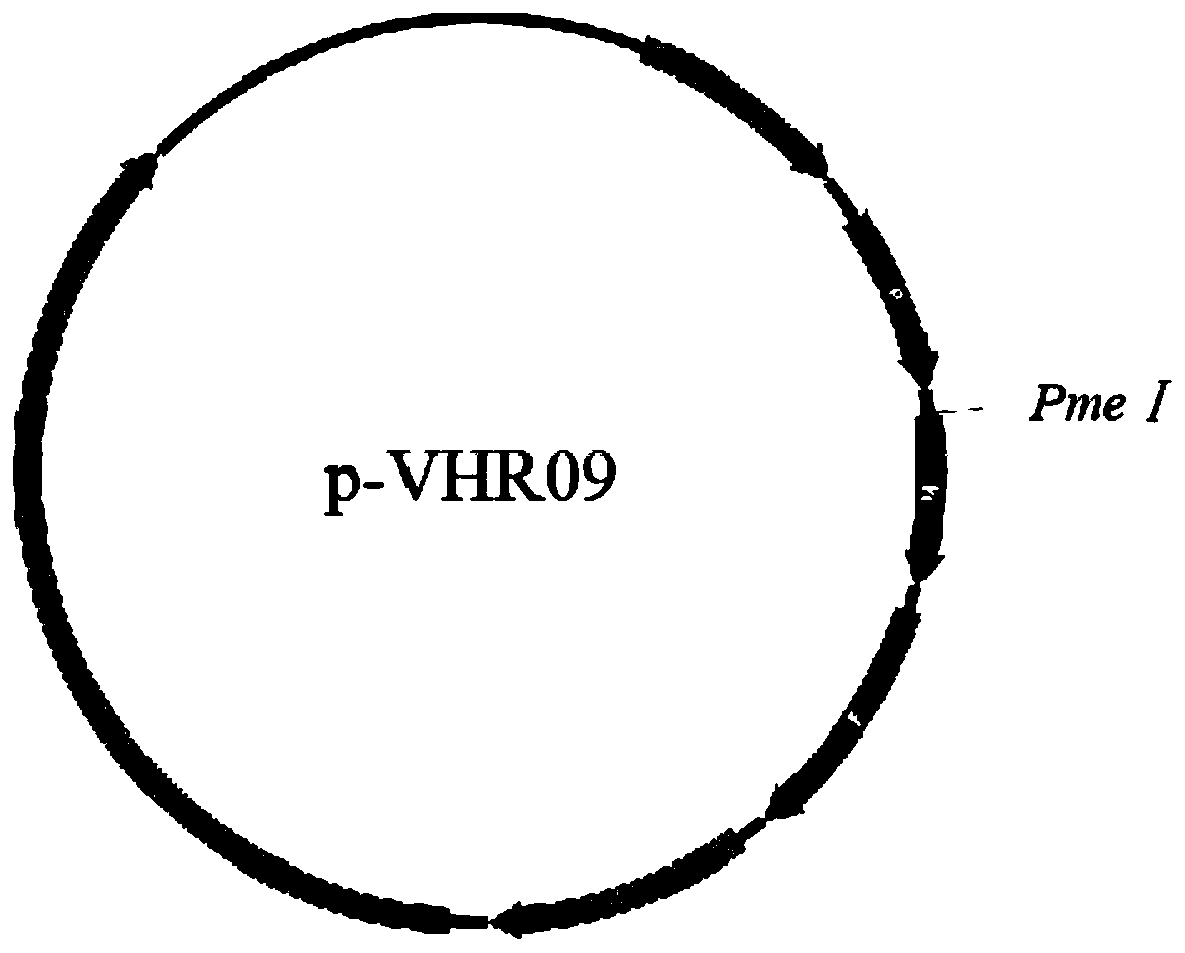
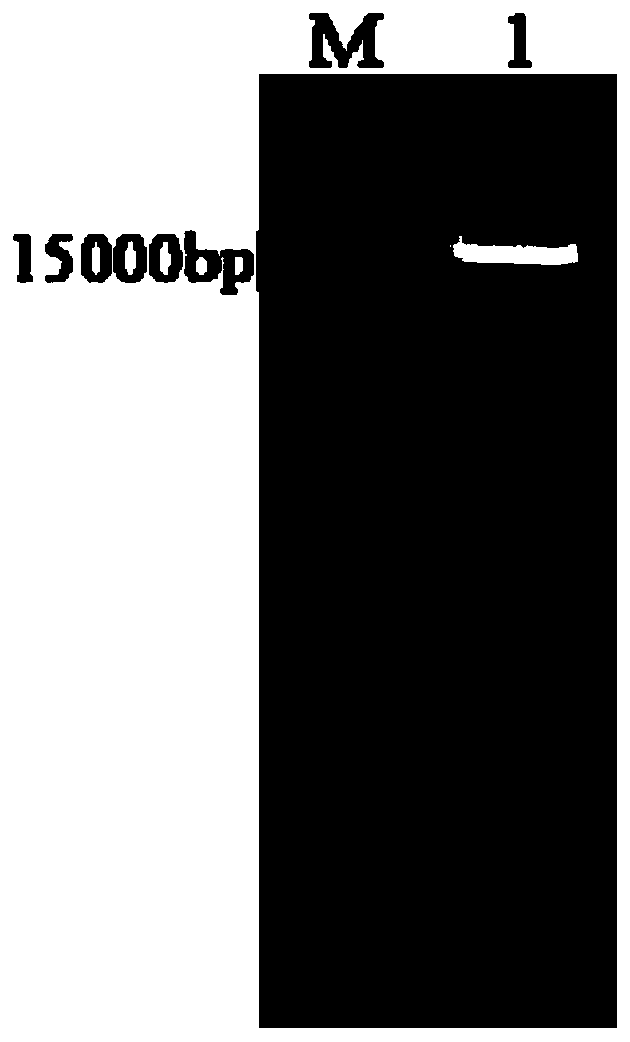
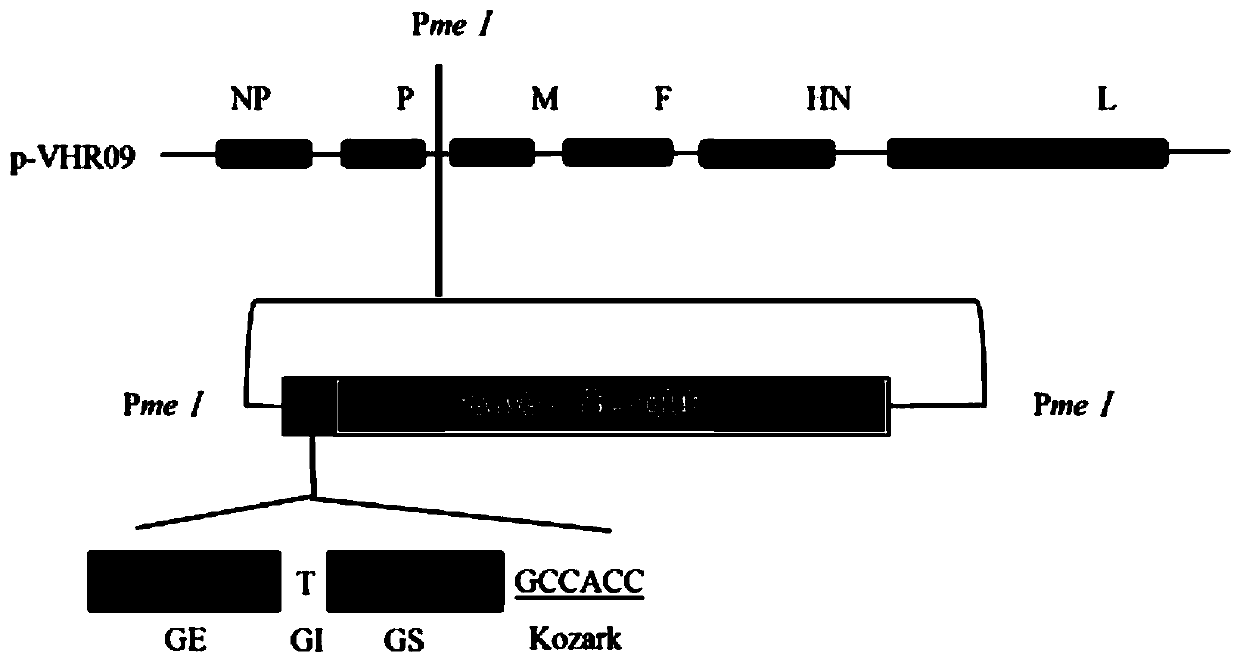
[0045] The transcription vector p-VHR09 containing the full-length genome of the attenuated NDV / rAHR09 mutant (GenBank: MF285077.1) was preserved by the Key Open Laboratory of Livestock and Poultry Infectious Diseases, Ministry of Agriculture, Yangzhou University (Cao Yongzhong et al. A thermostable gene Exogenous gene expression vector of type VIII attenuated Newcastle disease strain. Patent application number: CN201811244137.6, publication number: CN109295095A), see the plasmid map figure 1 . Use PmeI restriction endonuclease to carry out enzyme digestion and linearization in the intergenic region between P and M genes, and after the digestion product is subjected to 1% agarose gel electrophoresis, the linearized target fragment of about 18000bp is recovered, and the result is as follows figure 2 shown.
[0046] 2. Cloning and s...
Embodiment 2
[0067] Example 2: Identification of biological characteristics of recombinant virus
[0068] 1. Thermotolerance Determination of Recombinant Viruses
[0069] Take the 3rd generation allantoic fluid of the recombinant virus rAHR09-4F2, take 7 parts of 500 μL for each generation, put them in a metal bath at 56 ℃ for heat treatment, the heat treatment time is set to 20min, 30min, 40min, 50min, 60min, 70min, 80min, and carry out HA test. The results are shown in Table 1. The heat resistance test results show that rAHR09-4F2 still has HA activity after being treated at 56°C for 70 minutes.
[0070] Table 1 Determination results of heat-resistant hemagglutination activity of rAHR09-4F2 strain
[0071]
[0072] 2. EID of the recombinant virus 50 determination
[0073] The rAHR09-4F2 allantoic fluid was serially diluted 10 times, and 10 6 ~109 Doubly diluted virus, inoculate 9-11-day-old chicken embryos through the allantoic cavity, inoculate 5 eggs for each dilution of allant...
Embodiment 3
[0080] Embodiment 3: Recombinant virus rAHR09-4F2 immune efficacy test
[0081] 1. Experimental grouping and immunization
[0082] SPF chicken test groups are shown in Table 3. Vaccinations were performed when SPF chickens were 7 days old. The test chickens in the rAHR09-4F2 group were inoculated with rAHR09-4F2 (10 6 EID 50 ), the inactivated vaccine group was subcutaneously inoculated with FAdV-4+FAdV-8b bivalent inactivated vaccine (0.25mL / monkey) through the neck, and the challenge control group was inoculated with PBS (0.1mL / bird) through nasal drops. FAdV-4+FAdV-8b bivalent inactivated vaccine was prepared by referring to Hu Yage's method (Hu Yage. Development of bivalent oil emulsion inactivated vaccine of poultry group I adenovirus serotype 4 and 8b. Yangzhou University master's degree thesis, 2018.)
[0083] Table 3 Immunization test groups
[0084]
[0085] 2. Antibody level monitoring after immunization
[0086] On the 7th, 14th, 21st, and 28th day after th...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com