Application of calreticulin CALR to disease resistance of pigs
A protein and application technology, applied in the fields of application, antiviral agents, double-stranded DNA viruses, etc., can solve the problems of lack of effective molecular targets and unclear infection mechanism of JEV
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
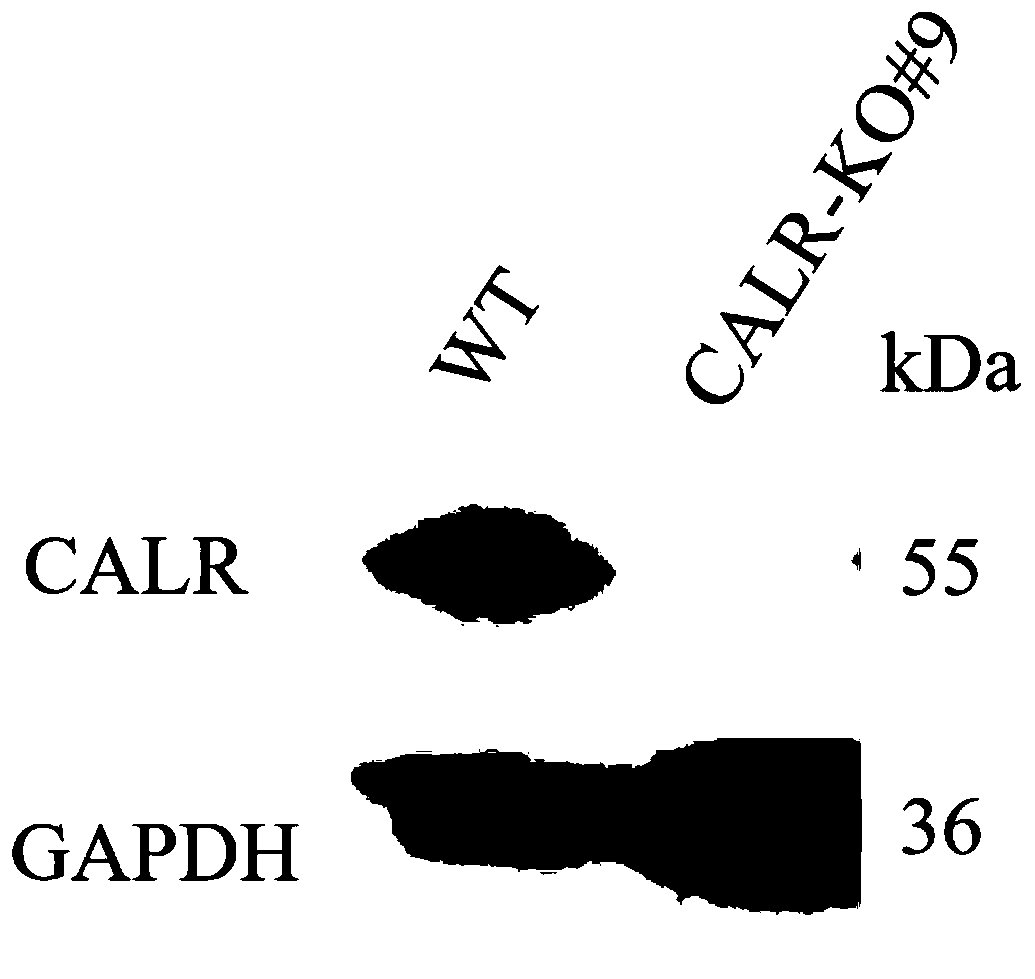
[0144] Example 1: Construction of porcine CALR gene knockout cell line using CRISPR / Cas9 lentivirus strategy
[0145] 1.1 Design of specific sgRNA targeting CALR gene and construction of expression vector
[0146] In order to study whether the CALR gene is involved in mediating JEV infection of pig PK-15 cells, the CALR gene (accession number: ENSSSCT00000015020) and the whole genome sequence of the pig (version number: Sus_scrofa.Sscrofa11) were downloaded from the Ensemble database (www.ensembl.org). .1). Then, use sgRNAcas9 software (https: / / sourceforge.net / projects / sgrnacas9 / ) to design sgRNA, and then select the sgRNA targeting the porcine CALR gene exon according to the specificity evaluation results. The sequence of the selected sgRNA is:
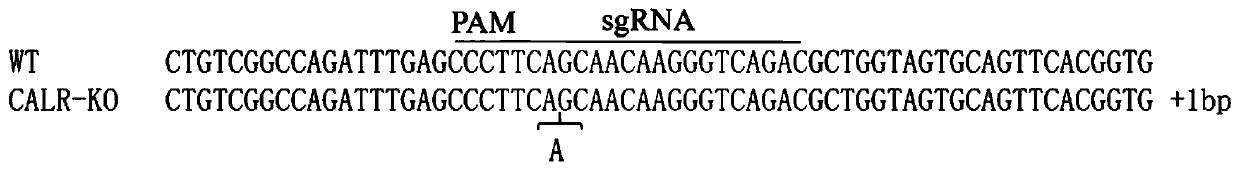
[0147] CALR-sgRNA 5'-GTCTGACCCTTGTTGCTGAA-3'
[0148] Among them, the PAM sequence for recognizing the target is "GGG". Genome-wide off-target assessment found no off-target sites with 1, 2 and 3 base mismatches ( ...
Embodiment 2
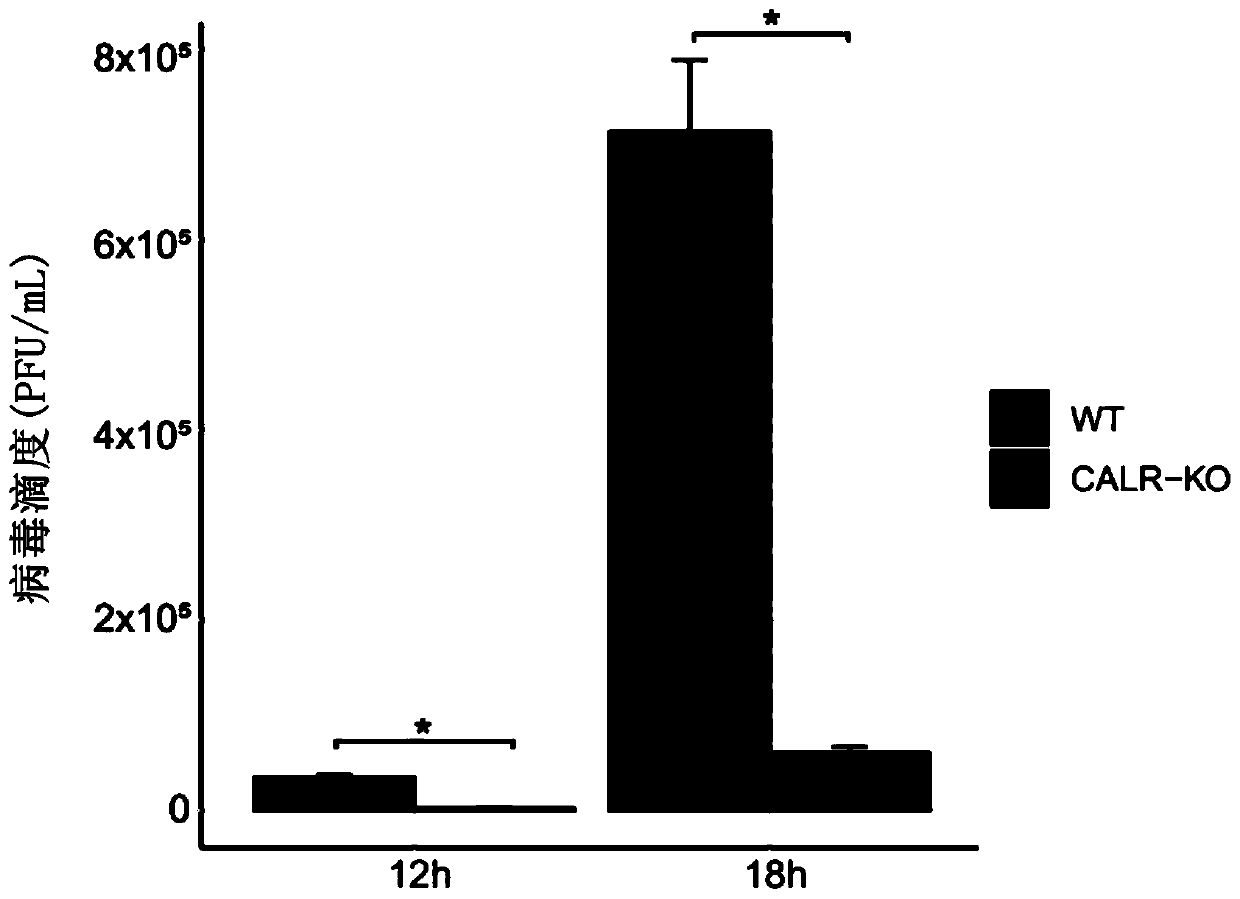
[0164]Example 2: It was found that CALR knockout cells can significantly inhibit the replication of JEV in pig PK-15 cells
[0165] 2.1 Plaque assay was used to detect the effect of CALR on JEV replication
[0166] The effect of CALR knockout cells on JEV replication was investigated using virus plaque assay and absolute quantification assay, respectively. The procedure of the virus plaque experiment is the low-melting point agarose method, and the operation is as follows: BHK cells are inoculated into a 6-well cell culture plate and cultivated to a monolayer. The cells were grown to a confluence of more than 50% for inoculation; preparation of virus dilution: take 100 μL of virus stock solution in a 1.5mL EP tube, and use high-glucose medium DMEM to carry out serial 10-fold dilutions (10 -1 , 10 -2 , 10 -3 , 10 -4 , 10 -5 , 10 -6 , 10 -7 , 10 -8 ), gently pipette to mix, and the number of pipettes should be the same for each tube. Be careful not to extend the tip of t...
Embodiment 3
[0176] Example 3: The NS3 gene encoded by JEV cannot be expressed in CALR gene knockout cells
[0177] The expression of NS3 gene after JEV infection in CALR gene knockout and control cells was further compared by immunofluorescence technique. The general experimental procedure is as follows: (1) Solution preparation: 0.3% TritonX-100 (ready to use); blocking solution: final concentration 3% BSA, 0.3% TritonX-100, 10% FBS, stored at 4°C. Antibody diluent: 3% BSA, 0.3% TritonX-100, stored at 4°C. (2) Operation process: Cell fixation: In a 12-well plate, when the cell density reaches 70%-80%, wash the cells twice with pre-cooled PBS, and then fix with pre-cooled 4% paraformaldehyde at room temperature 15min. Remove paraformaldehyde, rinse the cells twice with pre-cooled PBS, add pre-cooled 0.3% TritonX-100, and remove TritonX-100 after standing at room temperature for 10 minutes. Rinse cells: add pre-cooled PBS, incubate on a shaker at room temperature for 5 minutes, repeat t...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com