Preparation method of dimemorfan phosphate, and preparation methods of dimemorfan phosphate intermediates
A technology of dimethylorphinyl phosphate and dimethylarphane, which is applied in the field of medicine and chemical industry, can solve the problems of cumbersome filtration, extraction, pickling, alkali washing operation, complicated processing operation, affecting Grignard reaction, etc. Simple handling, simple reaction system, and the effect of improving reactivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
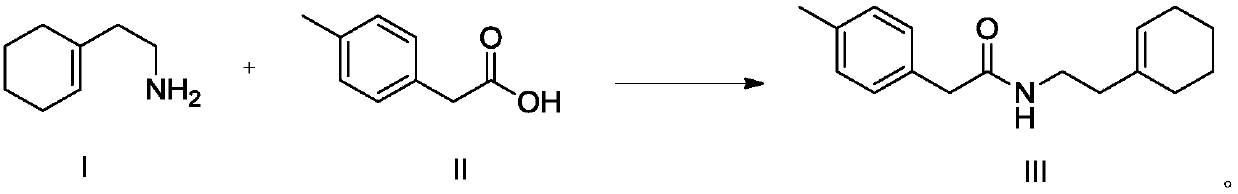
[0063] Preparation of 2-(4-methylphenyl)-N-[(cyclohexen-1-yl)ethyl]acetamide
[0064] The reaction formula is as follows:
[0065]
[0066] At room temperature, 15.00 g of 2-(1-cyclohexenyl)ethylamine, 14.39 g of p-tolueneacetic acid and 86 mL of toluene were sequentially added to a 250 mL one-necked bottle, and the reaction solution was heated to 60° C. for 30 h. The reaction of the raw materials was detected by HPLC, and the reaction solution was concentrated under reduced pressure at 55° C. to obtain 23.58 g of an orange oil with a yield of 95.6%, which was directly used in the next reaction without purification.
Embodiment 2
[0068] Preparation of 2-(4-methylphenyl)-N-[(cyclohexen-1-yl)ethyl]acetamide
[0069] The reaction formula is as follows:
[0070]
[0071] At room temperature, 15.00 g of 2-(1-cyclohexenyl)ethylamine, 53.97 g of p-tolueneacetic acid and 345 mL of toluene were sequentially added to a 250 mL one-necked bottle, and the reaction solution was heated to 150° C. for reflux and water separation for 10 h. The reaction of the raw materials was detected by HPLC, and the reaction solution was concentrated under reduced pressure at 55° C. to obtain 30.70 g of an orange oil with a yield of 99.6%, which was directly used in the next reaction without purification.
Embodiment 3
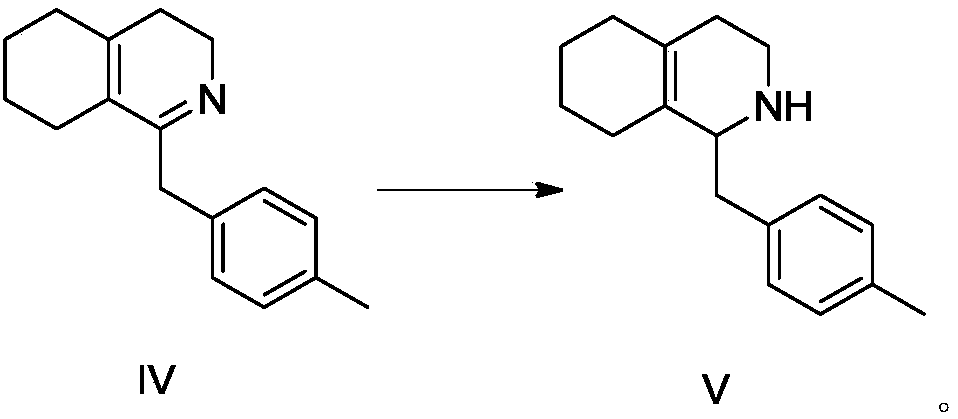
[0073] Preparation of 1-(4-methylbenzyl)-3,4,5,6,7,8 hexahydroisoquinoline
[0074] The reaction formula is as follows:
[0075]
[0076] At room temperature, 15.00 g of 2-(1-cyclohexenyl)ethylamine, 21.59 g of p-tolueneacetic acid and 150 mL of toluene were sequentially added to a 250 mL one-necked bottle, and the reaction solution was heated to 135° C. for 15 h under reflux and water separation. It was detected by HPLC that the reaction of the raw materials was complete, the temperature of the reaction solution was lowered to 80° C., and 50.51 g of phosphorus oxychloride was added to the reaction solution. The reaction solution was warmed up to 105°C and refluxed for 5 hours. After the reaction was completed, the reaction solution was concentrated under reduced pressure at 55° C. to obtain 28.50 g of an orange oil with a yield of 99.4%, which was directly used in the next reaction without purification.
PUM
| Property | Measurement | Unit |
|---|---|---|
| purity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com