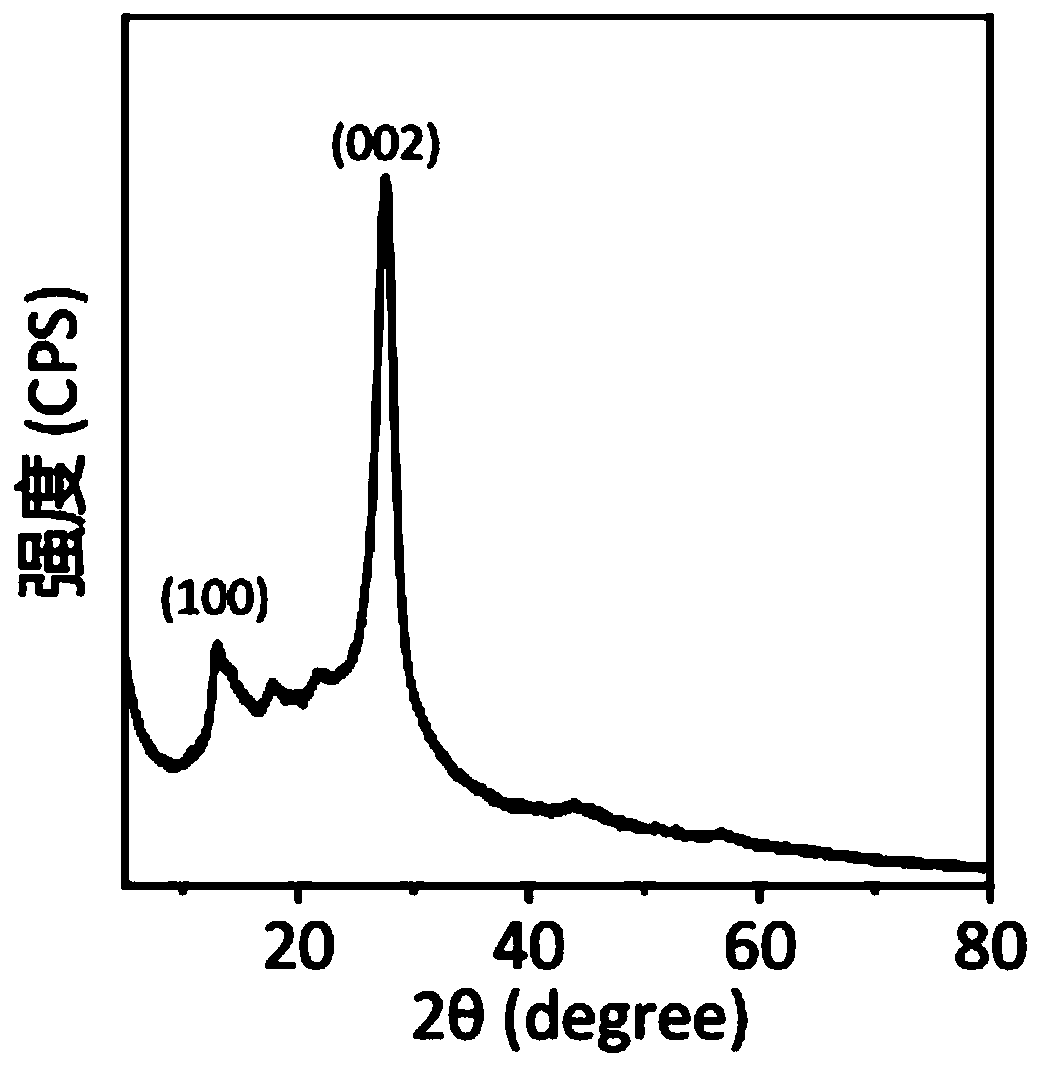
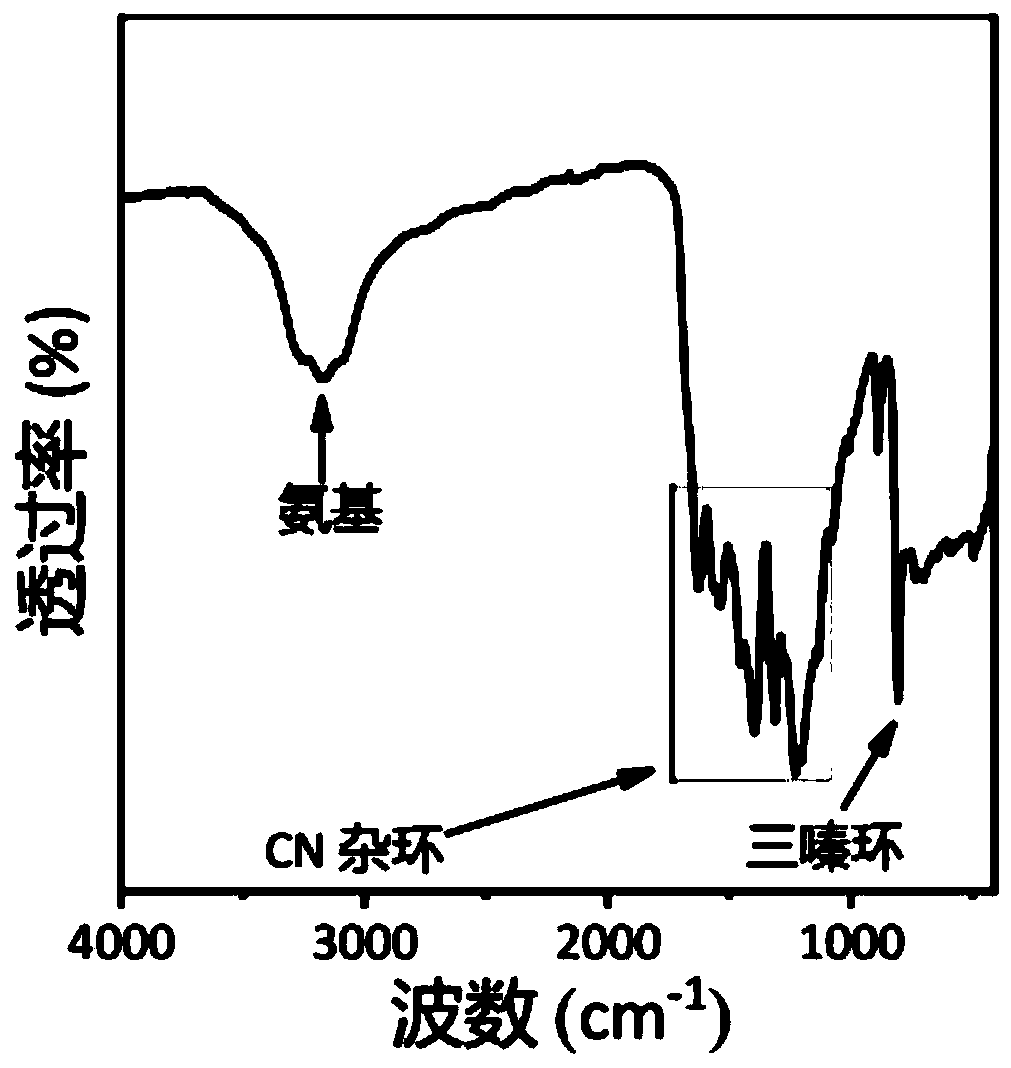
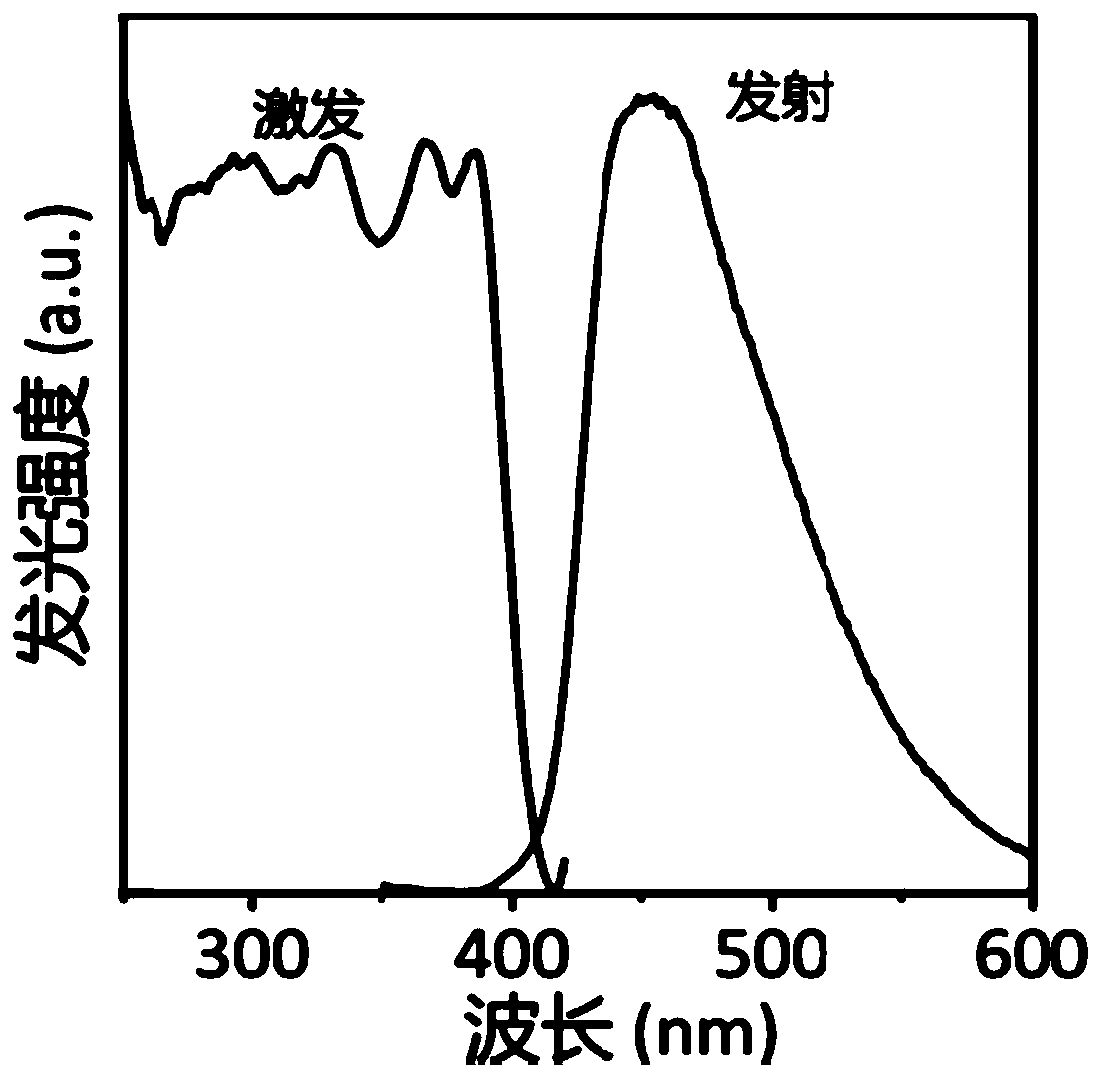
Carbon nitride paper-based fluorescence sensor for detecting polycyclic aromatic hydrocarbon, preparation method thereof and application of sensor
A technology of fluorescent sensor and carbon nitride paper, which is applied in the field of fluorescent sensing, can solve the problems of long detection period, complicated operation, and low stability of polycyclic aromatic hydrocarbons, and achieve the goal of improving optical stability, high sensitivity, and stable luminescent performance Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] The preparation process of carbon nitride nanocomposites is as follows:
[0047] Urea was raised to 550°C in a muffle furnace at a heating rate of 2°C / min and kept for 2 hours. After natural cooling at room temperature, CNNS was obtained directly, and 30mL of CNNS aqueous solution (0.2mg / mL) was prepared, and ultrasonic 30min (power 100W); Take by weighing 30mLPMAA-Na, 0.2β-CD and mix and stir with above-mentioned CNNS solution, carry out ultrasonic 15min (power is 100W); ) about 15mL, stir evenly, place the mixed solution in a water bath and heat at 80°C for 3 hours, the solution will produce PMAA-CNNS precipitation; after the temperature drops to room temperature, pour out the supernatant, and freeze the gelatinous precipitate at the bottom After drying, the obtained particles are collected for future use to obtain a carbon nitride nanocomposite material.
[0048] The preparation process of the carbon nitride paper-based fluorescent sensor is as follows:
[0049] Ta...
Embodiment 2
[0059] The preparation process of carbon nitride nanocomposites is as follows:
[0060] Dicyandiamide was raised to 550°C for 4 hours at a heating rate of 4°C / min in a muffle furnace. After natural cooling at room temperature, bulk carbon nitride was obtained, and the bulk carbon nitride was subjected to liquid-phase exfoliation by ultrasound for 12 hours. (power 100W) to obtain CNNS; prepare 30mL CNNS aqueous solution (0.5mg / mL), sonicate for 30min (power 100W); weigh 30mL PAM, mix 0.3gβ-CD with the above CNNS solution and stir evenly, and then sonicate for 15min (power 100W); the mixed solution was freeze-dried, and the obtained particles were collected for later use to obtain carbon nitride nanocomposites.
[0061] The preparation process of the carbon nitride paper-based fluorescent sensor is as follows:
[0062] Take 1g of the nanocomposite material and disperse it in 15mL of ethanol, cut the filter paper into 2*1cm 2 The filter paper was soaked in the solution for 20 m...
Embodiment 3
[0067] The preparation process of carbon nitride nanocomposites is as follows:
[0068] Melamine is heated up to 600°C for 4 hours at a heating rate of 4°C / min in a muffle furnace. After natural cooling at room temperature, bulk carbon nitride is obtained, and the bulk carbon nitride is subjected to ultrasonic 12-hour liquid phase stripping (power 100W ), to obtain CNNS; prepare 30mL CNNS aqueous solution (0.05mg / mL), ultrasonic 30min (power 100W); add 0.6g DMA and 0.05g MBA to the CNNS solution and mix, transfer the mixed solution to a vial, and pass nitrogen gas for 3- For 5 minutes, tighten the lid, and irradiate with a 100W fluorescent lamp until the gel is formed; freeze-dry the gel, collect the obtained particles for later use, and obtain a carbon nitride nanocomposite material.
[0069] The preparation process of the carbon nitride paper-based fluorescent sensor is as follows:
[0070] Take 1g nanocomposite and disperse in 20mL DMF, cut the filter paper into 2*1cm 2 s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com