Purpose of ATCA to preparation of medicines for tumors
An application and tumor technology, applied in the field of salts in the preparation of drugs for the treatment of tumors, can solve the problems of slowing down the translation and elongation rate of oncogenes, difficult to effectively inhibit the malignant phenotype of cancer cells, and limited use of puromycin.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
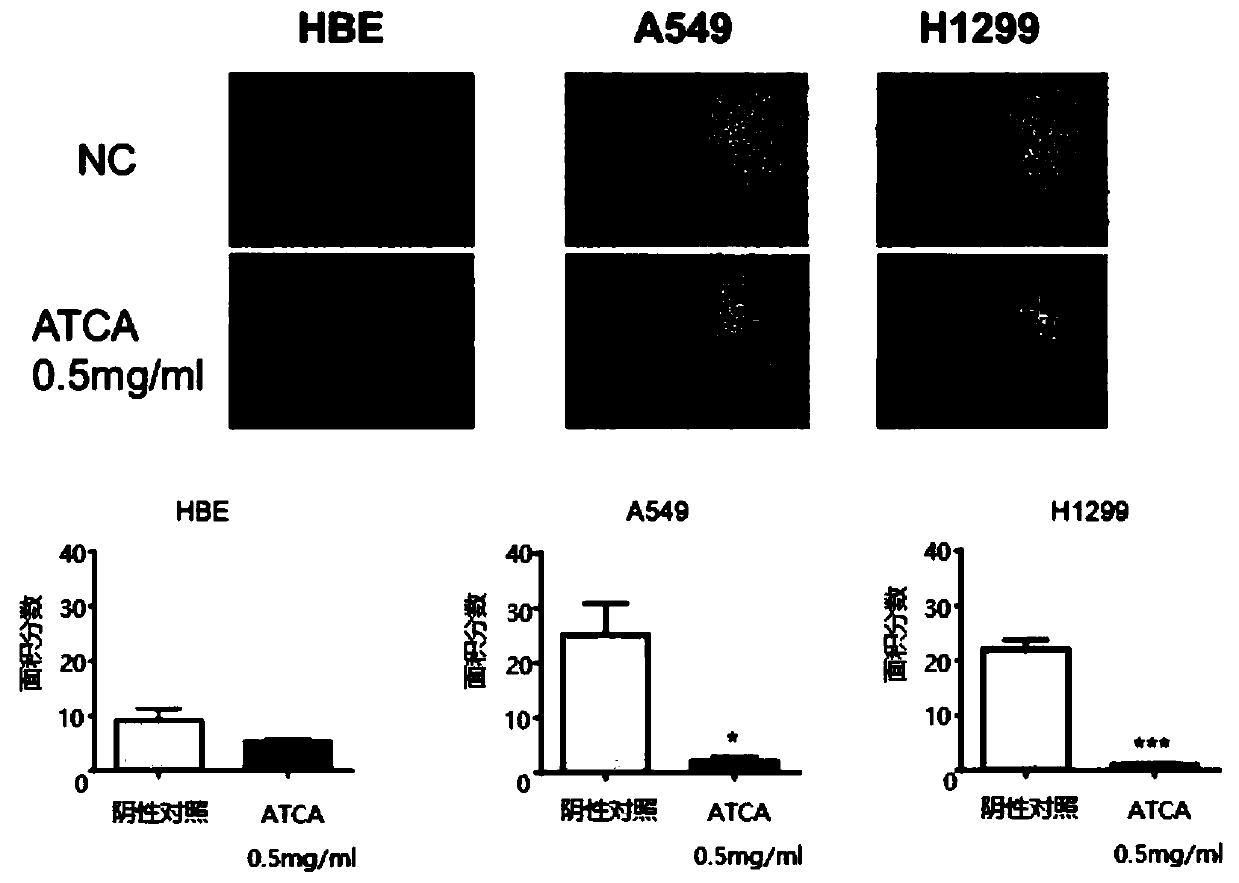
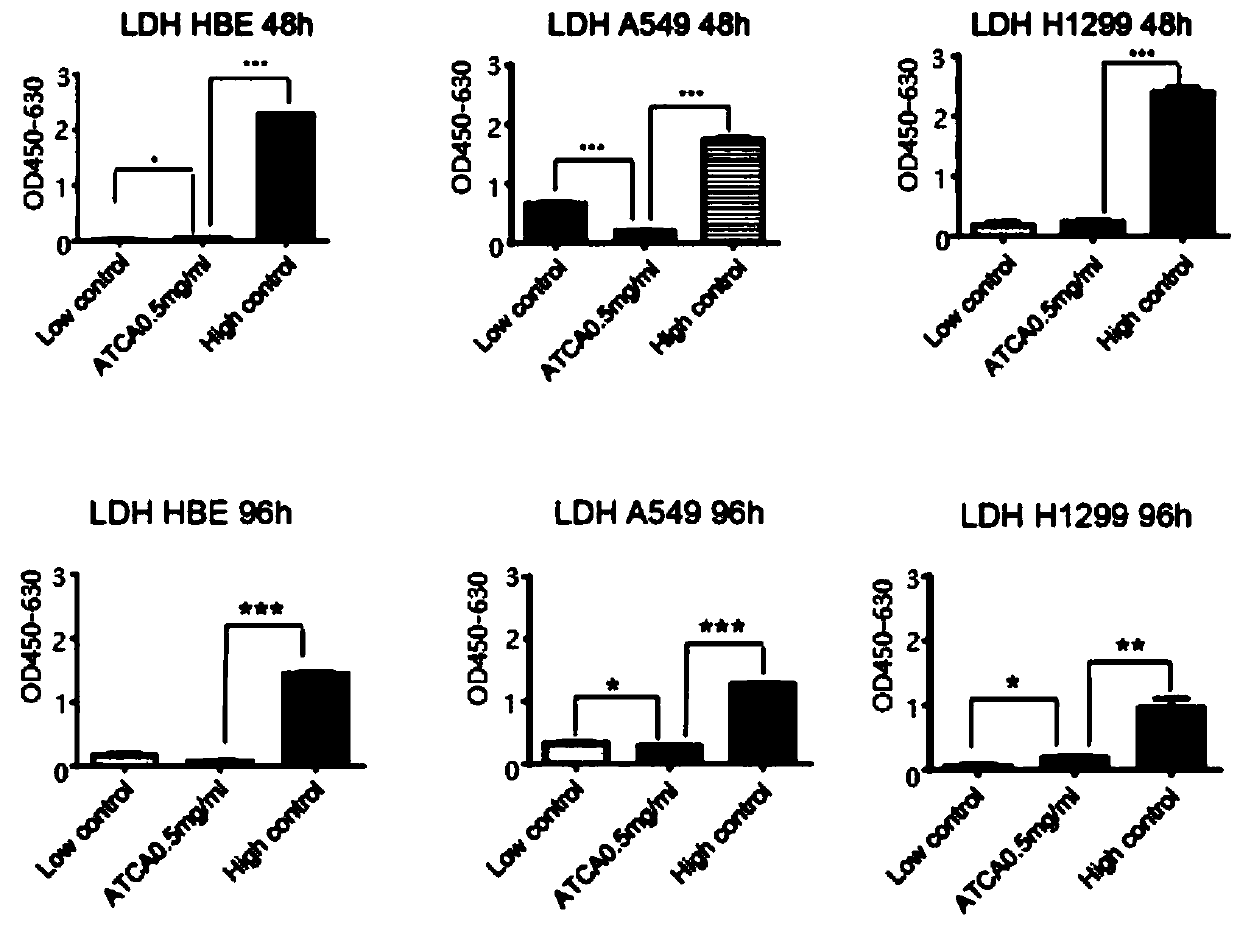
[0051] In order to test whether the method of the present invention has an inhibitory effect on the malignant phenotype of cancer cells and whether it can kill normal cells, experiments on cell proliferation, cell migration and cytotoxicity are carried out. The experimental steps are as follows:
[0052] (1) Use cell culture medium at 37°C, 0.5% CO 2 Culture human lung cancer cell lines A549, H1299 and lung normal epithelial cells HBE in the environment;
[0053] (2) Add ATCA solution so that the final concentration reaches 0.5mg / mL, 1.0mg / mL, 1.5mg / mL, 2.0mg / mL respectively, shake well, and continue to culture cells in the original environment.
[0054] Detection of cell proliferation, cell migration, and cytotoxicity in steps (1) and (2).
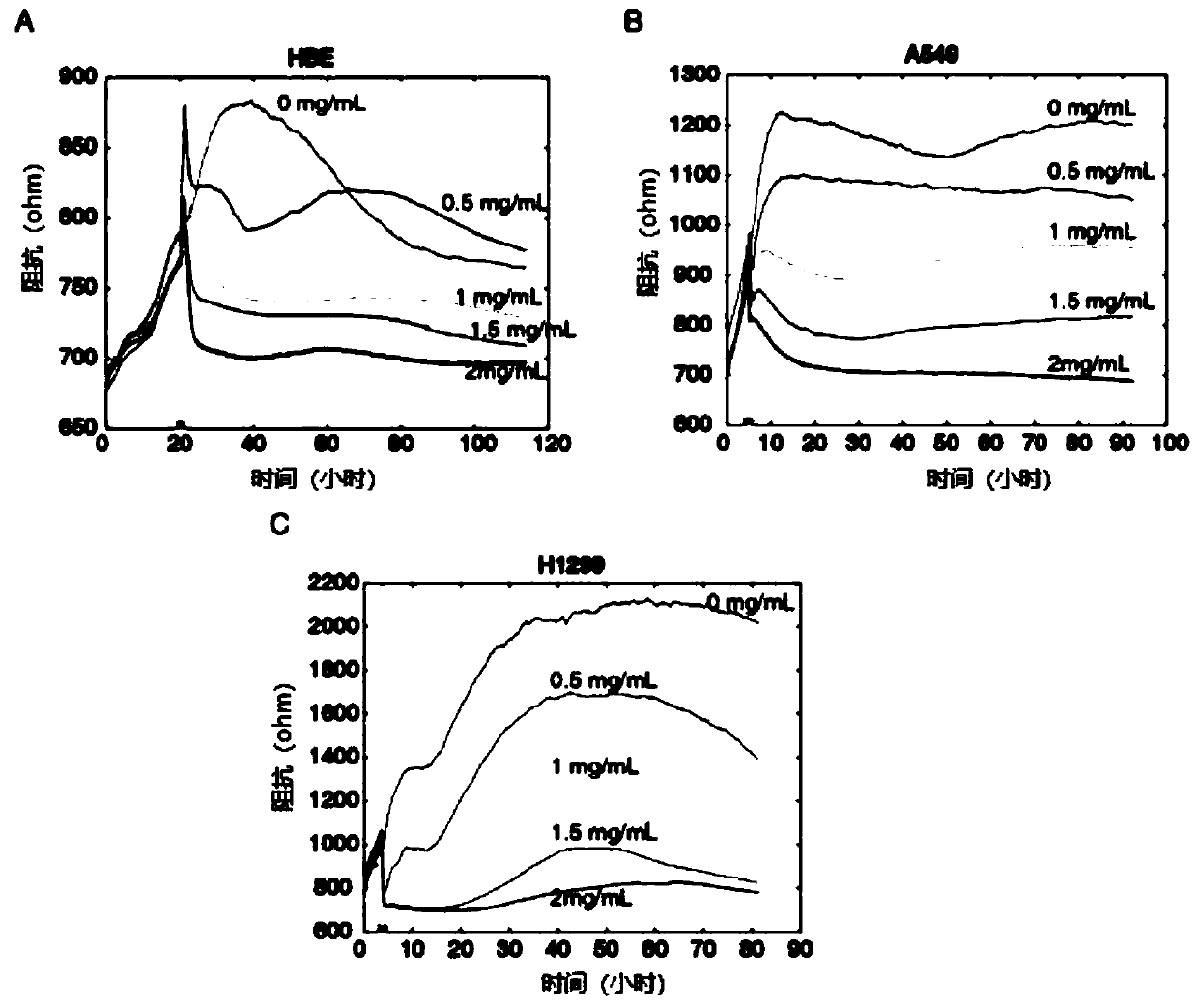
[0055] The cell proliferation results of HBE, A549 and H1299 cells before and after administration are as follows: figure 1 shown. Depend on figure 1 It can be seen that with the increase of drug concentration, the proliferation rate...
Embodiment 2
[0059] In order to check whether the method of the present invention has inhibitory effect on cancer cells and whether normal cells are killed, the following experiments are carried out to suppress the malignant phenotype of cancer cells on the nude mouse xenograft tumor model:
[0060] (1) Take nude mice and inject lung cancer cell A549 subcutaneously;
[0061] (2) Wait for nude mice to form tumors, and discard nude mice without tumors;
[0062] (3) The mice in the treatment group use 0.2mL of ATCA solution (concentration 25mg / mL) for intragastric administration, so that the drug dose of ATCA in each mouse reaches 5 mg, and intragastric administration once a day; mice in the control group use an equal volume of normal saline ( NaCl) gavage;
[0063] (4) At the same time, the nude mice not injected with cancer cells were taken as the healthy group, and the same amount of ATCA and NaCl solution as the experimental group was administered orally.
[0064] The survival curves, t...
Embodiment 3
[0069] In order to test whether the method of the present invention has an inhibitory effect on the malignant phenotype of lung cancer and other types of cancer cells, and whether it can kill normal cells, cell apoptosis and cell proliferation detection experiments are carried out. The experimental steps are as follows:
[0070] (1) Use cell culture medium at 37°C, 0.5% CO 2 Human lung cancer cell lines A549, H1299 and lung normal epithelial cells HBE, ovarian cancer cell line ES-2, liver cancer cell lines HCCLM3, HCCLM6 and MHCC97H were cultured in the environment;
[0071](2) Add ATCA solution, set the dosing concentration gradient, so that the final concentration reaches 0.125mg / mL, 0.25mg / mL, 0.5mg / mL, 0.75mg / mL, 1.0mg / mL, shake well, and continue in the original environment Cultured cells.
[0072] Use Annexin V and propidium iodide to stain human lung cancer cells A549, H1299 and normal lung epithelial cells for HBE, and then use flow cytometry to detect the results. ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com