Preparation method of micronutrient element zinc-manganese chelate
A technology of micronutrient and elemental zinc, which is applied in the field of preparation of zinc-manganese chelates, achieves the effect of simple method and increased content ratio
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
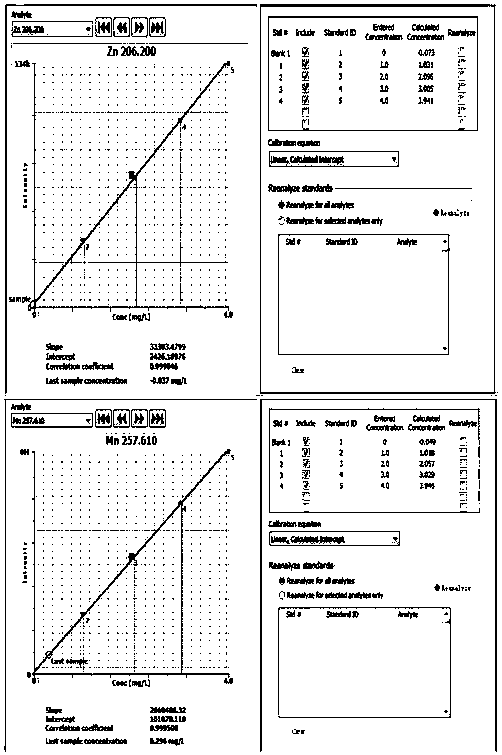
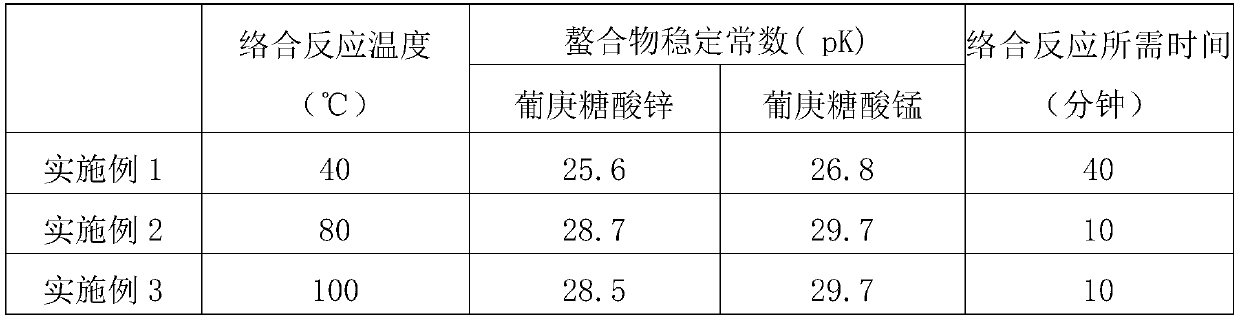
Image
Examples
Embodiment 1
[0038] 1) Preparation of aqueous solution of glucoheptononic acid
[0039] Solid sodium glucoheptonate (Wuhan Yuancheng Science and Technology Development Co., Ltd. industrial grade) was formulated into an aqueous solution, and the activated large-pore strong acid cation exchange resin (Shanghai Jinkai Resin Co., Ltd. D301) carried out ion exchange to obtain a mass percentage of 10% aqueous solution of glucoheptonic acid.
[0040] 2) Preparation of zinc hydroxide or manganese hydroxide
[0041] Preparation of zinc hydroxide: Stir saturated zinc sulfate solution, and dropwise add 0.2M sodium bicarbonate solution at the same time, stop dropping when white precipitate appears at pH 7.5-8; stop stirring and stand still for 24 hours; filter, wash, pump Just dry.
[0042] Preparation of manganese hydroxide: Stir the saturated manganese sulfate solution, and add dropwise a 0.2M sodium bicarbonate solution at the same time, stop the dropwise addition when a brown precipitate appears...
Embodiment 2
[0046] 1) Preparation of aqueous solution of glucoheptononic acid
[0047] Solid sodium glucoheptonate (Wuhan Yuancheng Science and Technology Development Co., Ltd. industrial grade) was formulated into an aqueous solution, and the activated large-pore strong acid cation exchange resin (Shanghai Jinkai Resin Co., Ltd. D301) carried out ion exchange to obtain a mass percentage of 20% aqueous solution of glucoheptonic acid.
[0048] 2) Preparation of zinc hydroxide or manganese hydroxide
[0049] Preparation of zinc hydroxide: Stir saturated zinc sulfate solution, and dropwise add 0.2M sodium bicarbonate solution at the same time, stop dropping when white precipitate appears at pH 7.5-8; stop stirring and stand still for 24 hours; filter, wash, pump Just dry.
[0050]Preparation of manganese hydroxide: Stir saturated manganese sulfate solution, and dropwise add 0.2M sodium bicarbonate solution at the same time, stop the dropwise addition when a brown precipitate appears above ...
Embodiment 3
[0054] 1) Preparation of aqueous solution of glucoheptononic acid
[0055] Solid sodium glucoheptonate (Wuhan Yuancheng Science and Technology Development Co., Ltd. industrial grade) was formulated into an aqueous solution, and the activated large-pore strong acid cation exchange resin (Shanghai Jinkai Resin Co., Ltd. D301) carried out ion exchange to obtain a mass percentage of 30% aqueous solution of glucoheptonic acid.
[0056] 2) Preparation of zinc hydroxide or manganese hydroxide
[0057] Preparation of zinc hydroxide: Stir saturated zinc sulfate solution, and dropwise add 0.2M sodium bicarbonate solution at the same time, stop dropping when white precipitate appears at pH 7.5-8; stop stirring and stand still for 24 hours; filter, wash, pump Just dry.
[0058] Preparation of manganese hydroxide: Stir saturated manganese sulfate solution, and dropwise add 0.2M sodium bicarbonate solution at the same time, stop the dropwise addition when a brown precipitate appears above...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com