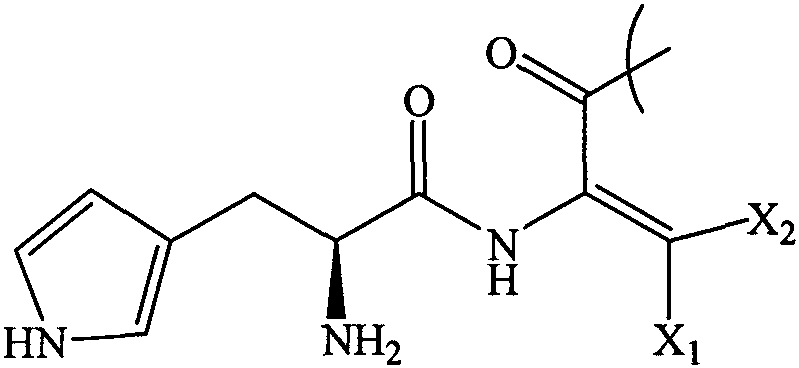
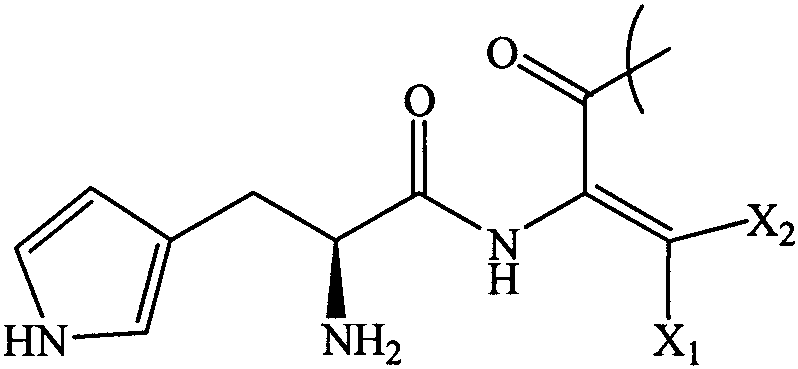
Tiripa peptide analogue
A technology of ripatide and analogues, applied in the field of tiripatide analogues, can solve the problems of inability to achieve full-effect blood sugar control and weight loss, and achieve the effects of stable properties, weight loss, and weight loss
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1 Preparation of Compound 1
[0039] Tyr-Dhthr-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Tyr-Ser-Ile-Dhthr-
[0040] Leu-Asp-Lys-Ile-Ala-Gln-Lys (AEEA-AEEA-γGlu-18 alkanedioic acid)-
[0041] Ala-Phe-Val-Gln-Trp-Leu-Ile-Ala-Gly-Gly-Pro-Ser-Ser-Gly-
[0042] Ala-Pro-Pro-Pro-Ser-NH 2
[0043] The preparation method includes: preparing the peptide resin by solid-phase polypeptide synthesis method, acid hydrolyzing the peptide resin to obtain a crude product, and finally purifying the crude product to obtain a pure product; wherein the step of preparing the peptide resin by the solid-phase polypeptide synthesis method is to pass solid-phase The phase-coupled synthesis method sequentially inserts the corresponding protected amino acids or fragments in the following sequences to prepare peptide resins:
[0044] In the above preparation method, the amount of the Fmoc-protected amino acid or protected amino acid fragment is 1.2-6 times of the total moles of the resin fed; prefera...
Embodiment 2
[0078] Example 2 Preparation of Compound 2
[0079] Tyr-Dhval-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Tyr-Ser-Ile-Dhval-
[0080] Leu-Asp-Lys-Ile-Ala-Gln-Lys (AEEA-AEEA-γ-Glu-18 alkanedioic acid)-
[0081] Ala-Phe-Val-Gln-Trp-Leu-Ile-Ala-Gly-Gly-Pro-Ser-Ser-Gly-
[0082] Ala-Pro-Pro-Pro-Ser-NH 2
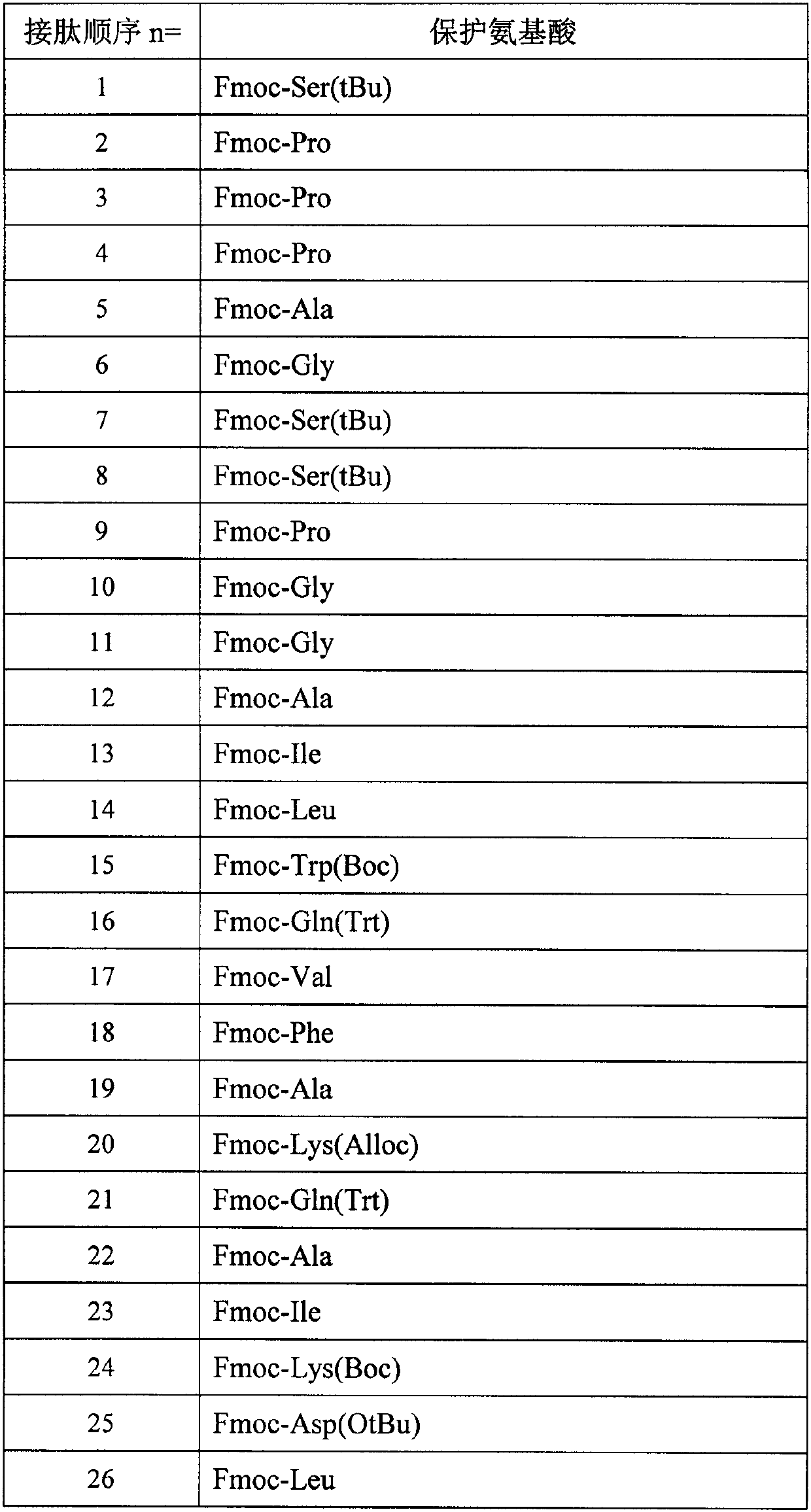
[0083] The preparation method is the same as in Example 1, and the protected amino acids used are as follows:
[0084]
[0085]
[0086] 6.1 g of pure product was obtained, the purity was 96.9%, and the total yield was 12.5%. Molecular weight 4865.2 (100% M+H).
Embodiment 3
[0087] Example 3 Preparation of compound 3
[0088] Tyr-Dhthr-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Tyr-Ser-Ile-Dhthr-
[0089] Leu-Asp-Lys-Ile-Ala-Gln-Lys (PEG 5 CH2 CO-γGlu-18 alkanedioic acid)-
[0090] Ala-Phe-Val-Gln-Trp-Leu-Ile-Ala-Gly-Gly-Pro-Ser-Ser-Gly-
[0091] Ala-Pro-Pro-Pro-Ser-NH 2
[0092] The preparation method is the same as in Example 1, and the protected amino acids used are as follows:
[0093]
[0094]
[0095] 7.4 g of pure product was obtained, the purity was 96.3%, and the total yield was 15.3%. Molecular weight 4824.8 (100% M+H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com