Preparation method of p-acetoxystyrene
A technology of acetoxystyrene and acetoxyacetophenone, which is applied in the field of preparation of p-acetoxystyrene, can solve the problems of complex process, low yield, expensive reagents, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0049] The invention provides a kind of preparation method of acetoxystyrene, comprising:
[0050] 1) reacting p-hydroxyacetophenone with an acetylating reagent to prepare p-acetoxyacetophenone;
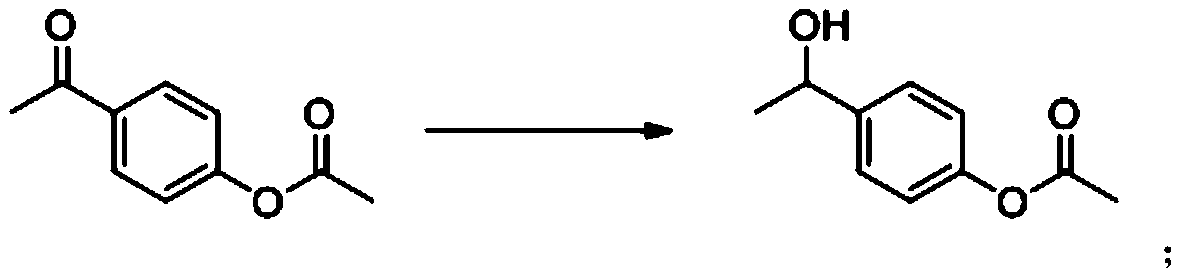
[0051] 2) Hydrogenating and reducing p-acetoxyacetophenone to prepare 1-(4-acetoxyphenyl)ethanol;
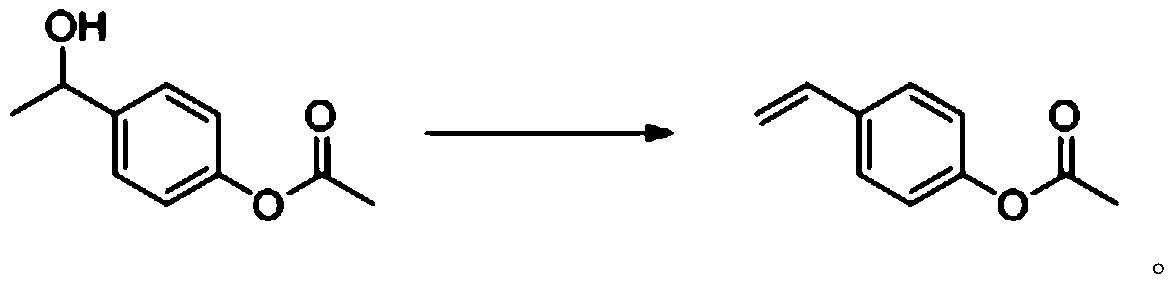
[0052] 3) Eliminate 1-(4-acetoxyphenyl)ethanol to prepare p-acetoxystyrene.
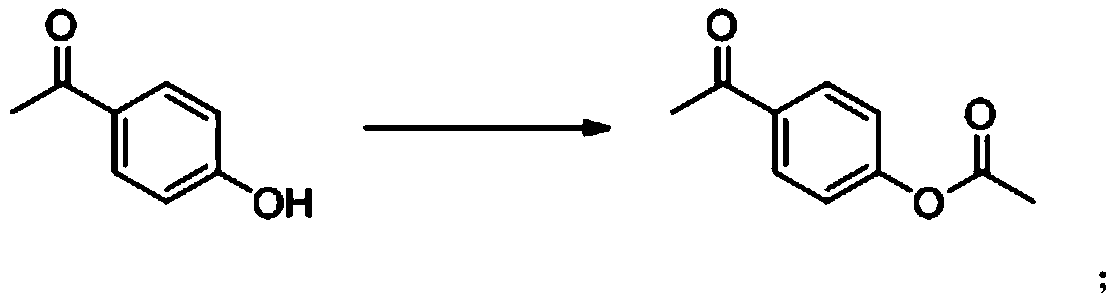
[0053] The preparation method of acetoxystyrene provided by the present invention may include an acetylation reaction, and the acetylation reaction may specifically include: reacting p-hydroxyacetophenone with an acetylating reagent to prepare p-acetoxyacetophenone , the reaction equation is as follows:
[0054]
[0055] In the present invention, in the acetylation reaction, the reaction is usually carried out in the presence of an acid-binding agent, thereby providing alkaline reaction conditions and neutralizing the acid generated during the reaction. Those skilled in the art can select the appropriat...
Embodiment 1
[0077] Synthesis of p-acetoxyacetophenone:
[0078] In a 10L reaction flask, add 4080g of ethyl acetate, 1360g of p-hydroxyacetophenone, and 1272g of sodium carbonate in sequence, start stirring, and add 1335g of acetyl chloride dropwise at a temperature of 20-30°C. After the dropwise addition was completed, continue to insulate and stir for 8 hours, filter, and the filtrate was washed and layered with 3022g of 8% aqueous sodium bicarbonate solution, and the organic layer was distilled to reclaim the solvent, and 1380g of hexane was added to the concentrated residue, filtered after stirring and crystallizing, and filtered. After the cake was dried, 1660 g of p-acetoxyacetophenone was obtained, and the HPLC purity was >99.9%. 1 H NMR (400MHz, (CD 3 ) 2 SO): δ = 2.31 (s, 3H), 2.59 (s, 3H), 7.29 (d, J = 8.8, 2H), 8.02 (d, J = 8.8, 2H). 13 C NMR (100MHz, (CD 3 ) 2 SO): δ=21.34, 27.18, 122.58, 128.99, 134.89, 154.59, 169.31, 197.36.
Embodiment 2
[0080] Synthesis of p-acetoxyacetophenone:
[0081] In a 1000mL reaction flask, add 390g of dichloromethane, 130g of p-hydroxyacetophenone, and 116g of triethylamine in sequence, start stirring, and add 108g of acetyl chloride dropwise at a temperature of 30-40°C. After the dropwise addition was completed, the insulation and stirring were continued for 6 hours, then 260 g of water was added, and after stirring for 0.5 hours, layers were separated. 130g of water was added to the organic layer, and adjusted to neutral with sodium bicarbonate, the solvent was recovered by distillation of the layered organic layer, 130g of hexane was added to the concentrated residue, stirred and crystallized, and then filtered, and the filter cake was dried to obtain 150g of para-acetoxy Acetophenone, HPLC purity 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com