Novel electrochemical method of detecting copper ions, phosphate ions and alkaline phosphatase and application thereof
A technology of phosphate ion and copper ion, which is applied in the field of functional biomaterials and biosensing to achieve accurate results, less time-consuming, and high-sensitivity detection
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] The preparation of embodiment 1 sensor
[0036] The technical solution adopted by the present invention to solve the above technical problems is: a novel electrochemical method and application for detecting copper ions, phosphate ions and alkaline phosphatase, the specific steps are as follows:
[0037] Preparation of Electrode 1:
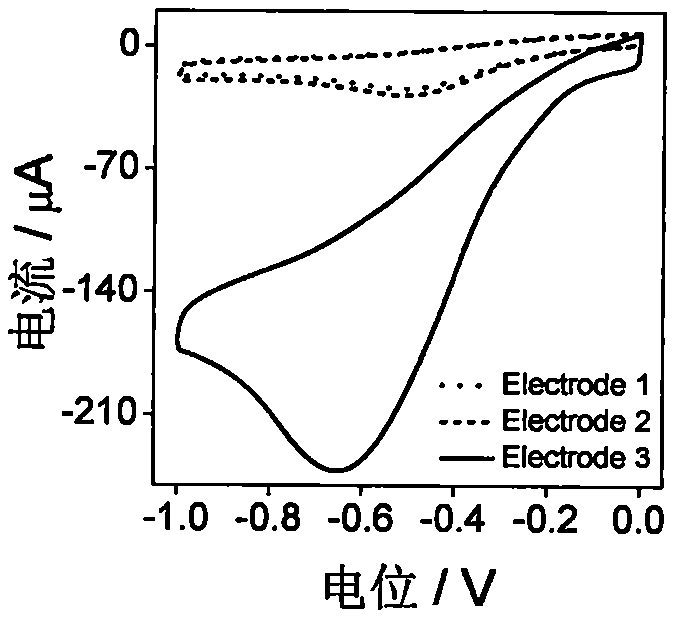
[0038] First, the glassy carbon electrode (GCE, 3 mm in diameter) was polished on the suede with Al2O3 powder (0.05 μm) for 5 min. N 2 Blow dry to get a bare glassy carbon electrode. The electrode is recorded as Electrode 1;
[0039] Preparation of Electrode 2:
[0040] Graphene was dispersed in HAc-NaAc (0.2M, pH 5.0) buffer solution, the control concentration was 1.0mg·mL -1 , Ultrasonic dispersion 2h. Place the bare GCE electrode in the above solution, and use cyclic voltammetry at a potential range of -1.5V to +0.5V at 10mV s -1 The sweep speed scans 10 laps. Get the GO modified electrode, which is denoted as Electrode 2;
[004...
Embodiment 2
[0045] The detection of embodiment 2 Cu(II)
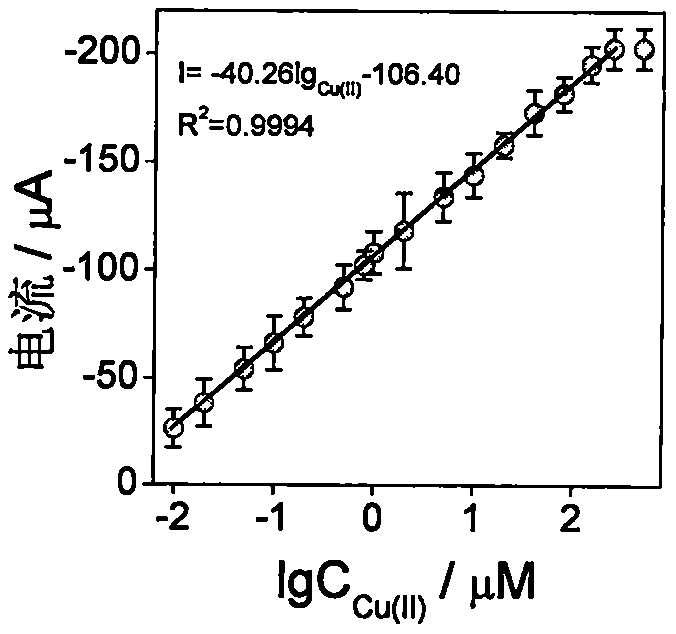
[0046] In the preparation process of Example 1 Electrode 3, the concentration of Cu(II) was changed, and the final concentration of Cu(II) was controlled to be: 0, 0.01, 0.02, 0.05, 0.1, 0.2, 0.5, 0.8, 1, 2, 5, 10, 20, 40, 80, 150, 250, 500 μM, a series of electrochemical biosensors were prepared as in Example 1. Experimental results such as figure 2 As shown, within a certain range, the greater the target concentration, the more obvious the current response. The linear correlation equation of the current response of the sensor to the logarithmic value of the Cu(II) concentration is I=-40.26lg Cu(II) -106.40, R 2 =0.9994, the linear range is 0.01-250 μM, and the detection limit is 0.0021 μM, indicating that the sensor can achieve highly sensitive detection of Cu(II) concentration.
Embodiment 3
[0047] The detection of embodiment 3 PPi
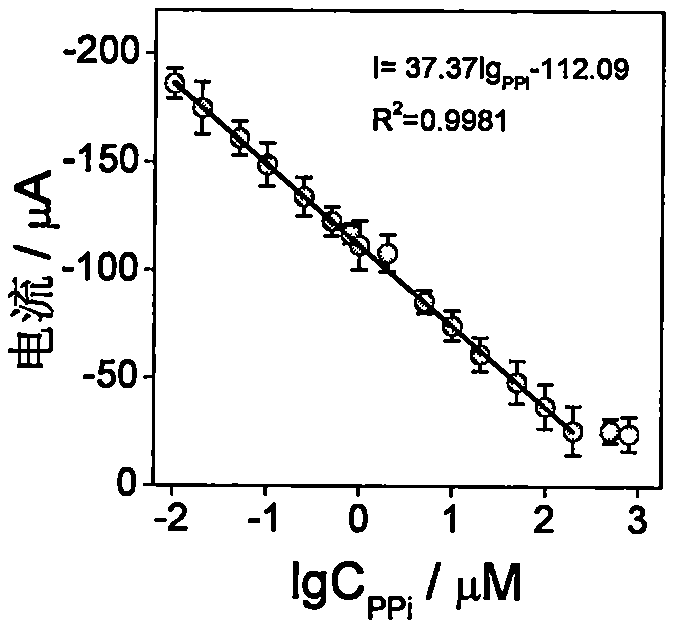
[0048] During the preparation process of Example 1 Electrode 3, the concentration of PPi was changed to control the final concentration of PPi to be respectively: 0, 0.01, 0.02, 0.05, 0.1, 0.25, 0.5, 0.8, 1, 2, 5, 10, 20, 50, 100, 200, 500, 800 μM, a series of electrochemical biosensors were prepared as in Example 1. Experimental results such as image 3 As shown, within a certain range, the greater the target concentration, the lower the current response, which proves that PPi forms a complex with Cu(II). The current response of the sensor to the linear correlation equation of the logarithmic value of the PPi concentration is I=37.37lg PPi -112.09, R 2 =0.9981, the linear range is 0.01-200 μM, and the linear correlation equation shows that the detection limit is 0.0035 μM. It shows that the sensor can realize highly sensitive detection of PPi concentration.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com