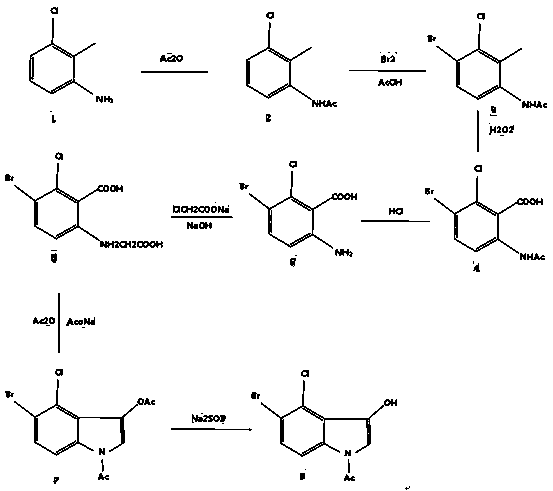
Method for synthesizing 5-bromine-4-chlorine-1-acetyl-3-indoxyl
A synthetic method, the technology of acetyl group, applied in the direction of organic chemistry, etc., can solve the problems of complex reaction process, difficult control, low total yield, etc., and achieve the effect of simple and practical process, easy to obtain, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] The first step is to add 200 grams of acetic anhydride to a 500ml three-necked flask, keep the temperature at about 5°C, stir evenly and slowly add 24 grams of 3-chloro-2-methylaniline, and keep the temperature for 12 hours after the addition is complete. , followed by TLC, when the reaction of 3-chloro-2-methylaniline was completed, 32 grams of water were added to hydrolyze acetic anhydride to obtain an acetic acid solution of 3-chloro-2-methylacetaniline. (The yield of this part is 90.6%)
[0030] The second step is to dissolve 14 grams of bromine in 42 grams of glacial acetic acid, and add it dropwise to another three-necked flask that is not concentrated and crystallized (containing 3-chloro-2-methylacetanilide) at a rate of one drop per minute. Acetic acid solution), use an ice-water bath to control the temperature in the three-necked flask between -5∽0°C, add dropwise after 4 hours, use TLC to track, pour into 1000ml ice water after the reaction is completed, stir...
Embodiment 2
[0037] The first step, add 100 grams of acetic anhydride to a 500ml three-necked flask, keep the temperature at about 5°C, slowly add 12 grams of 3-chloro-2-methylaniline under stirring conditions, and keep the temperature after the addition The reaction was carried out for 12 hours, followed by TLC. When the reaction of 3-chloro-2-methylaniline was completed, 16 g of water was added to hydrolyze acetic anhydride to obtain an acetic acid solution of 3-chloro-2-methylacetaniline. (The yield of this part is 90.6%)
[0038] The second step is to dissolve 7 grams of bromine in 21 grams of glacial acetic acid, and add it dropwise to another three-necked flask that is not concentrated and crystallized (containing 3-chloro-2-methylacetanilide) at a rate of one drop per minute. Acetic acid solution), use an ice-water bath to control the temperature in the three-necked flask between -5∽0°C, add dropwise after 4 hours, use TLC to track, pour into 1000ml ice water after the reaction is c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com