Medicinally and edibly homogenous sugar-lowering granule and preparation method and application thereof
A medicine and food homology, hypoglycemic technology, applied in the field of medicine and health care, can solve the problems of unsuitable long-term use, large side effects, lack of coordination, etc., and achieve the effects of reducing scattering, convenient taking, and convenient transportation and carrying
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
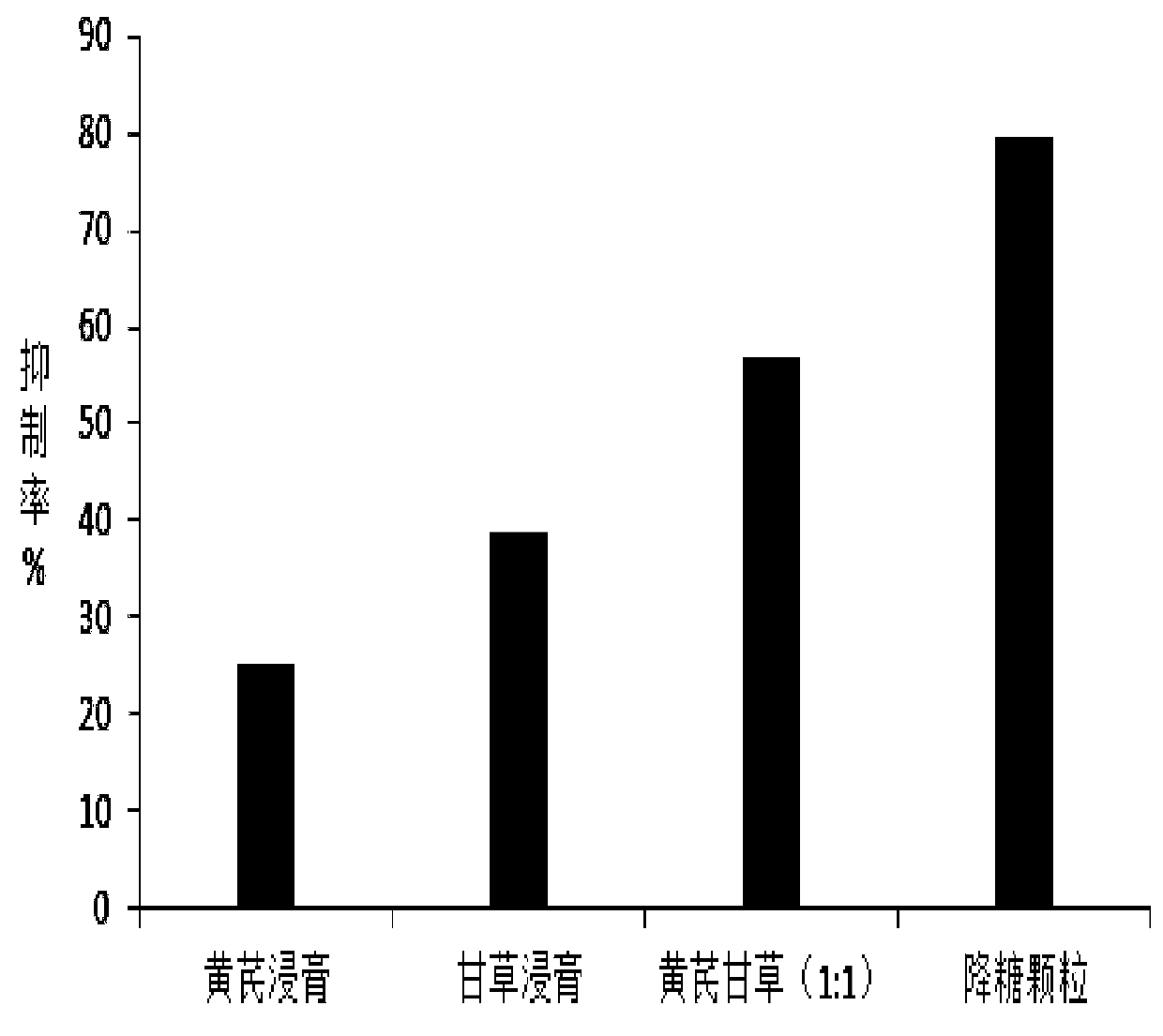
Image
Examples
Embodiment 1
[0049] A hypoglycemic granule with the same medicine and food, comprising the following raw materials in parts by weight: 25g of astragalus and licorice extract; 8g of pumpkin powder; 8g of sweet potato powder; 8g of bitter melon powder; 42g of soluble starch; 7g of maltodextrin; sodium citrate 0.2g; potassium sorbate 0.1g; xylitol 1.7g.
[0050] Its preparation method comprises the following steps:
[0051] (1) Cut fresh pumpkin, sweet potato and bitter melon into 2-3cm 3 Put the small pieces into an oven at 75°C to dry for 6.5 hours, with a water content of <5%; put them into a pulverizer to pulverize them, and then pass the obtained component powders through a 150-mesh sieve to make the particle size 90± 4.6μm;
[0052] (2) Mix astragalus and licorice at a mass ratio of 1:1, and then mix them with drinking water at a ratio of 1:10-15 to a volume ratio of solid to liquid, soak for 3.5 hours, then heat and extract at 95°C, Extract twice, 2.5 hours each time, add 15 times o...
Embodiment 2
[0055] A hypoglycemic granule with the same medicine and food, comprising the following raw materials in parts by weight: 22g of astragalus and licorice extract; 8g of pumpkin powder; 7g of sweet potato powder; 7g of bitter melon powder; 8g of maltodextrin; 46g of soluble starch; potassium sorbate 0.1g; sodium citrate 0.2g; xylitol 1.7g.
[0056] Its preparation method comprises the following steps:
[0057] (1) Cut fresh pumpkin, sweet potato and bitter melon into 2-3cm 3 Put the small pieces into an oven at 80°C to dry for 6 hours, with a water content of <5%; put them into a pulverizer to pulverize them, and then pass the obtained component powders through a 150-mesh sieve to make the particle size 90±4.6 μm;
[0058] (2) Mix astragalus and licorice at a mass ratio of 1:1, and then mix them with drinking water at a ratio of 1:10-15 to a volume ratio of solid to liquid. After soaking for 3 hours, heat and extract at 90°C to extract Twice, 2 hours each time, add 15 times o...
Embodiment 3
[0061] A hypoglycemic granule with the same medicine and food, comprising the following raw materials in parts by weight: 25g of astragalus and licorice extract; 7g of pumpkin powder; 10g of sweet potato powder; 9g of bitter melon powder; 7g of maltodextrin; 40g of soluble starch; potassium sorbate 0.1g; sodium citrate 0.2g; xylitol 1.7g.
[0062] Its preparation method comprises the following steps:
[0063] (1) Cut fresh pumpkin, sweet potato and bitter melon into 2-3cm 3 Put the small pieces in an oven at 85°C for 7 hours, and the water content is <5%; put them into a pulverizer to pulverize them, and then pass the obtained component powders through a 150-mesh sieve, so that the particle size is 90±4.6 μm;
[0064] (2) Mix astragalus and licorice at a mass ratio of 1:1, and then mix them with drinking water at a ratio of 1:10-15 to a volume ratio of solid to liquid. After soaking for 4 hours, heat and extract at 100°C to extract Twice, 2 hours each time, add 15 times of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com