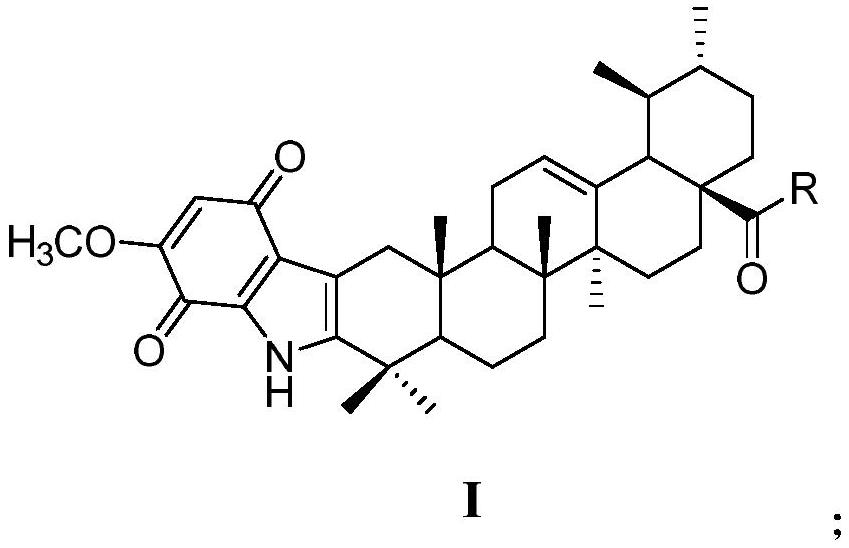
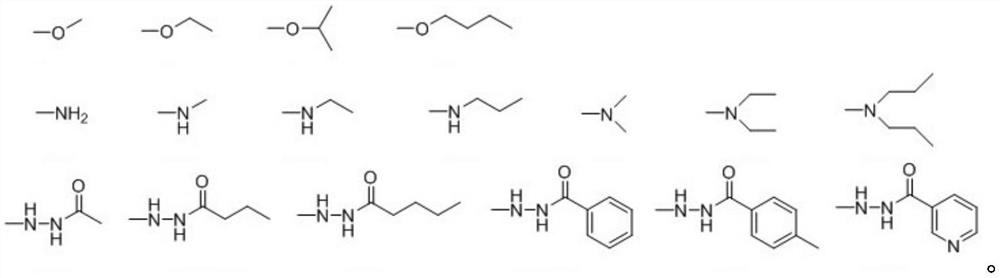
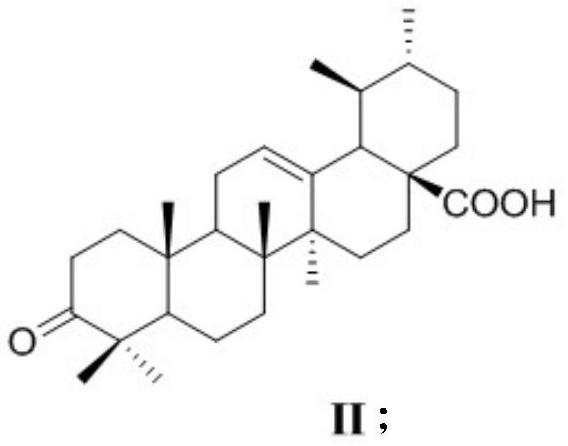
A kind of ursolic acid indole quinone derivatives and preparation method and application thereof
A technology of aldehyde indole derivatives and dimethoxyindole derivatives, which is applied in the field of ursolic acid indole quinone derivatives and their preparation, can solve the problems of lack of anti-tumor activity and achieve novel structures Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Embodiment 1 (1) synthetic 3-oxidized ursolic acid (II):
[0034] Add 4.6mmol of ursolic acid and 250mL of acetone into a 500mL round bottom flask, stir to dissolve, then stir in ice water for 15min, slowly add 1.87mL of Jones reagent dropwise and rise to room temperature, stir for 5h, then add 90mL of isopropanol Stirring and reacting for 30min, after the reaction was finished, the precipitate was filtered to collect the filtrate, the light yellow-green viscous solid obtained by concentrating the filtrate under reduced pressure was recrystallized with methanol, and the white needle-like crystal obtained was 3-oxyursolic acid (II) (1.2g, 65.6%).
[0035]
[0036] (2) Synthesis of ursolic acid 3,5-dimethoxindole derivative (III):
[0037] Weigh 3mmol of 3,5-dimethoxyaniline and dissolve it in 3mL of 20% hydrochloric acid in a single-necked round bottom flask. Then weigh 4 mmol of sodium nitrite and dissolve it in 0.7 mL of water, slowly drop it into the reaction fla...
Embodiment 2
[0050] Synthesis of indolequinone ursolic acid ester (I-b):
[0051] Dissolve 0.054 g of compound V in Example 1 in benzene, slowly drop 50 μL of SOCl 2 Afterwards, the temperature was gradually raised to 80°C, and the reaction was refluxed for 4 hours. When the wetted pH test paper is placed in the exhaust port, the test paper does not turn red, indicating that the reaction is over, concentrate under reduced pressure, and remove the benzene and SOCl in the reaction solution. 2 After steaming, ursolic acid indole quinone acid chloride was obtained in the form of yellow oil. Dissolve ursolic acid indoloquinone chloride with 6 mL of diethyl ether and slowly add 0.225 mmol of ethanol and 30 μL of triethylamine in dichloromethane dropwise under ice-bath conditions, slowly rise to room temperature and stir for 6 h. After the reaction, the reaction solution was poured into a mixture of ice and water, and extracted three times with dichloromethane after the ice melted; the combined...
Embodiment 3
[0054] Synthesis of indolequinone ursolic acid ester (I-c):
[0055]Dissolve 0.054 g of compound V in Example 1 in benzene, slowly drop 50 μL of SOCl 2 Afterwards, the temperature was gradually raised to 80°C, and the reaction was refluxed for 4 hours. When the wetted pH test paper is placed in the exhaust port, the test paper does not turn red, indicating that the reaction is over, concentrate under reduced pressure, and remove the benzene and SOCl in the reaction solution. 2 After steaming, ursolic acid indole quinone acid chloride was obtained in the form of yellow oil. Dissolve ursolic acid indoloquinone chloride with 6 mL of ether and slowly add 0.225 mmol of isopropanol and 30 μL of triethylamine in methylene chloride solution dropwise under ice-bath conditions, slowly rise to room temperature and stir for 6 h. After the reaction, the reaction solution was poured into a mixture of ice and water, and extracted three times with dichloromethane after the ice melted; the c...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com