Expression and purification method of recombinant human haptoglobin beta subunit protein
A technology of haptoglobin and expression method, which is applied in the field of expression and purification of recombinant human haptoglobin β subunit protein, can solve the problems of haptoglobin reduction, increase of free hemoglobin concentration, unfavorable blood transfusion patients, etc., and achieve simple operation , low cost effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Construction of recombinant expression vector pET32a(+)-HPβ
[0027] 1.1 Using chemically synthesized DNA template and PCR method to obtain human haptoglobin β subunit gene
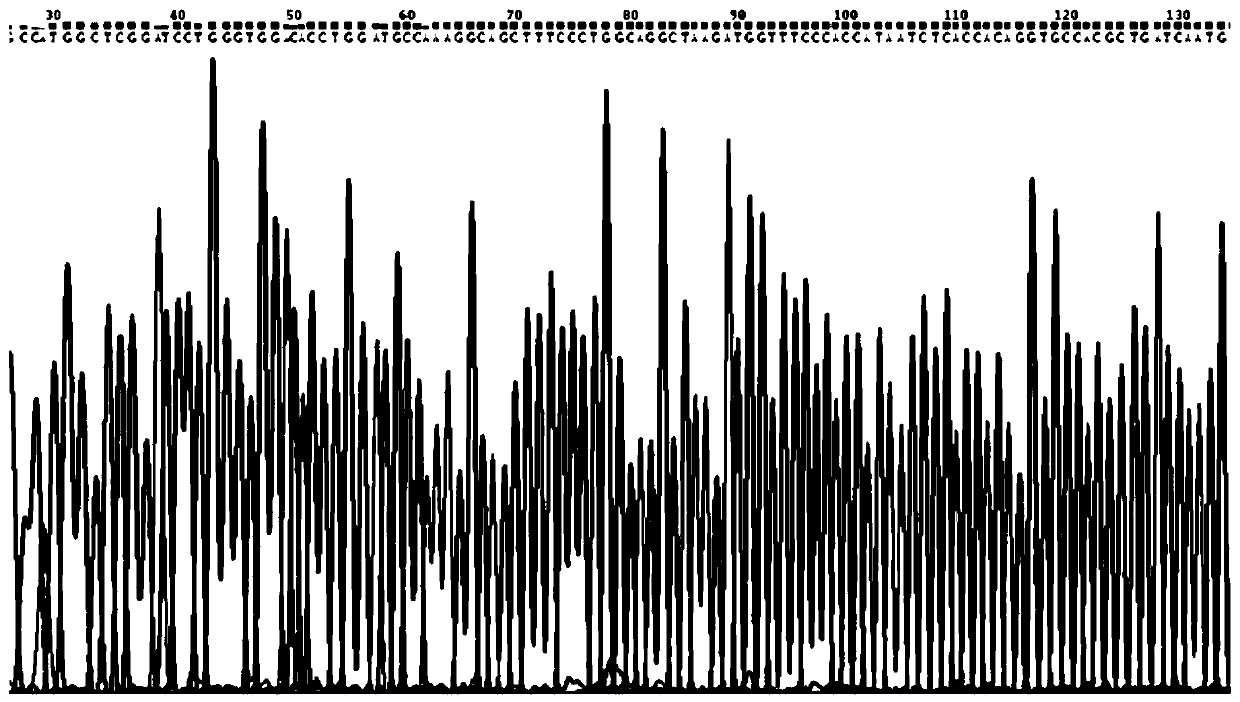
[0028] The human haptoglobin DNA template was synthesized in General Biosystems (Anhui) Co., Ltd. and inserted into the pUC57 cloning vector. The sequence length is 1062 bp, as shown in the appendix SEQ NO.1. Using this as a template, a full-length gene fragment of human haptoglobin β subunit was amplified. Its upstream primer sequence is: CAAGG CCATGG CTCGGATCCTGGGTGGACACC (the underlined part is the Nco I restriction site); its downstream primer sequence is:
[0029] GTG CTCGAG TTAGTTCTCAGCTATGGTCTTC (the underlined part is Xho I restriction site). PCR reaction system: 1μL upstream and downstream primers, 0.2μL plasmid template, 2xPfu PCR MasterMix (Tiangen Biochemical Technology Co., Ltd., KP201) 12.5μL, ddH 2 O 10.3μL; 94°C pre-denaturation for 3min; 94°C denaturation for 30s, 55°C annealing for 30...
Embodiment 2
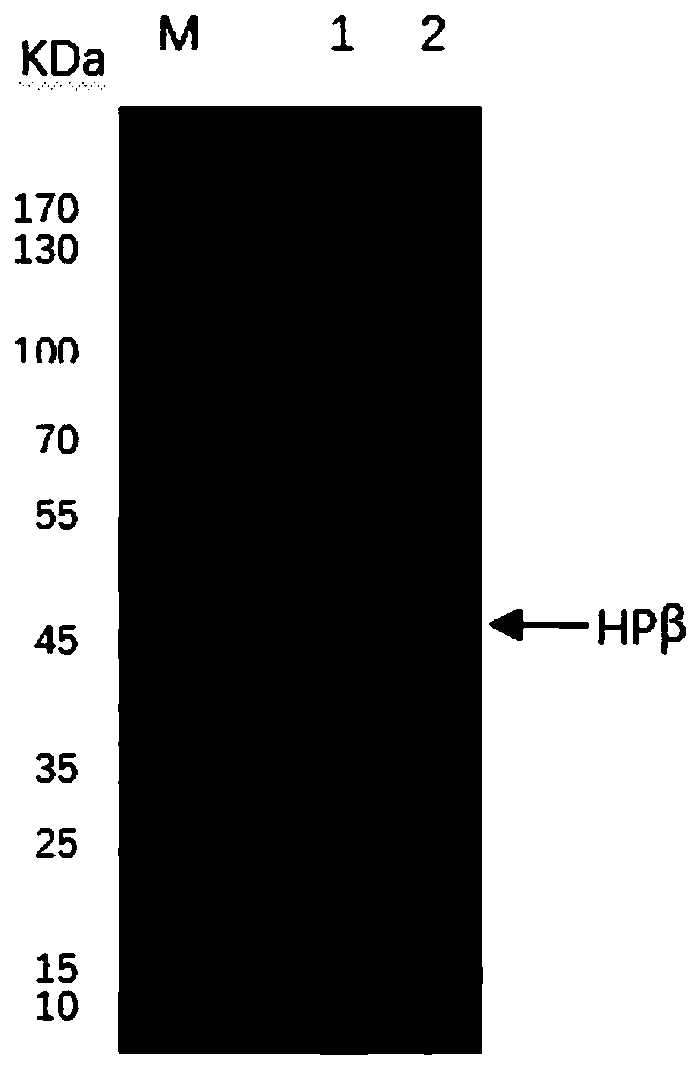
[0035] Expression and identification of recombinant human haptoglobin
[0036] 2.1 Pick a single colony of recombinant genetically engineered strain Shuffle T7-B-pET32a(+)-HPβ and inoculate it in 10ml LB medium (containing 100μg / mL of ampicillin), and culture overnight at 37°C with shaking at 150rpm.
[0037] 2.2 Inoculate the overnight cultured bacterial solution into 10ml fresh LB medium (containing 100μg / mL ampicillin) at a ratio of 1:9,
[0038] Incubate at 37°C and 150rpm for about 3 hours until the OD600 of the bacterial solution reaches 0.5-0.7, and when the OD600 is preferably close to 0.6, add IPTG with a final concentration of 1 mM to induce expression for 4 hours, and the expressed recombinant human haptoglobin is soluble protein and A mixture of inclusion bodies. At the same time, the engineered strain ShuffleT7-B-pET32a(+)-HPβ did not undergo IPTG induction group for control.
[0039] 2.3 After the induction culture, the culture solution was centrifuged at 12,000g for 1 ...
Embodiment 3
[0041] Purification of recombinant human haptoglobin β subunit protein
[0042] 3.1 According to the method of Example 2, induce the expression of 200mL of recombinant genetic engineering strain ShuffleT7-B-pET32a(+)-HPβ, resuspend the bacteria in an appropriate amount of pre-cooled PBS buffer, and ultrasonically break in an ice bath until the bacteria liquid turns from turbid to clear Centrifuge at 12,000g for 5 min at 4°C, and collect the supernatant. Add the supernatant solution to an equal volume of pre-cooled Soluble Binding Buffer (20mMTris-HCL (pH7.9), 500mMNaCL, 10mM imidazole), and use Kangwei's His Tag Protein Purification Kit (soluble protein) for purification.
[0043] 3.2 Load the supernatant containing soluble protein equal-fold diluted with Soluble Binding Buffer onto a Ni Sepharose chromatography column, first wash the column with 15 times the column volume of Soluble Binding Buffer, and then use Soluble with 500 mM imidazole concentration Elution Buffer (20mM Tris...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com