Diphenyl ether chalcone tubulin inhibitor as well as preparation method and application thereof
A technology of tubulin inhibition and chalcone class, applied in the preparation of carbon-based compounds, chemical instruments and methods, preparation of organic compounds, etc., can solve the problems of difficult synthesis and preparation, large side effects, complex structure, etc. Low, simple preparation method, the effect of inhibiting tubulin polymerization
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
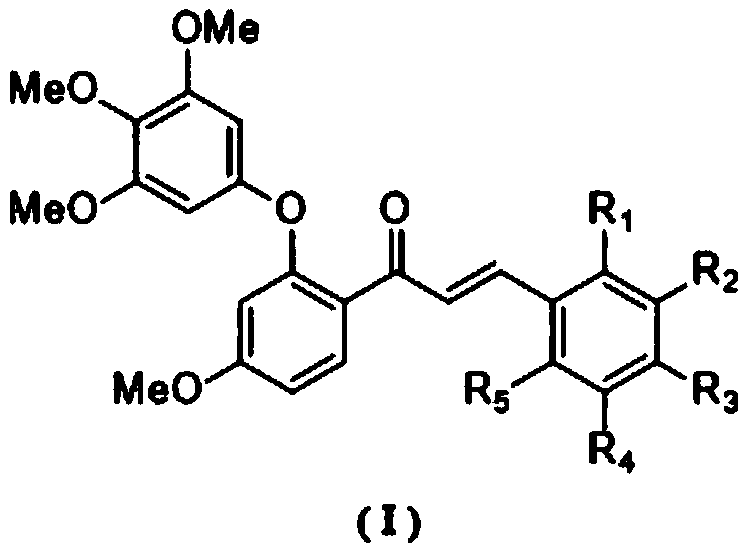
[0026] Example 1: (E)-3-(4-chlorophenyl)-1-(4-methoxy-2-(3,4,5-trimethoxyphenoxy)phenyl)propane-2- Preparation of en-1-one (1)
[0027]
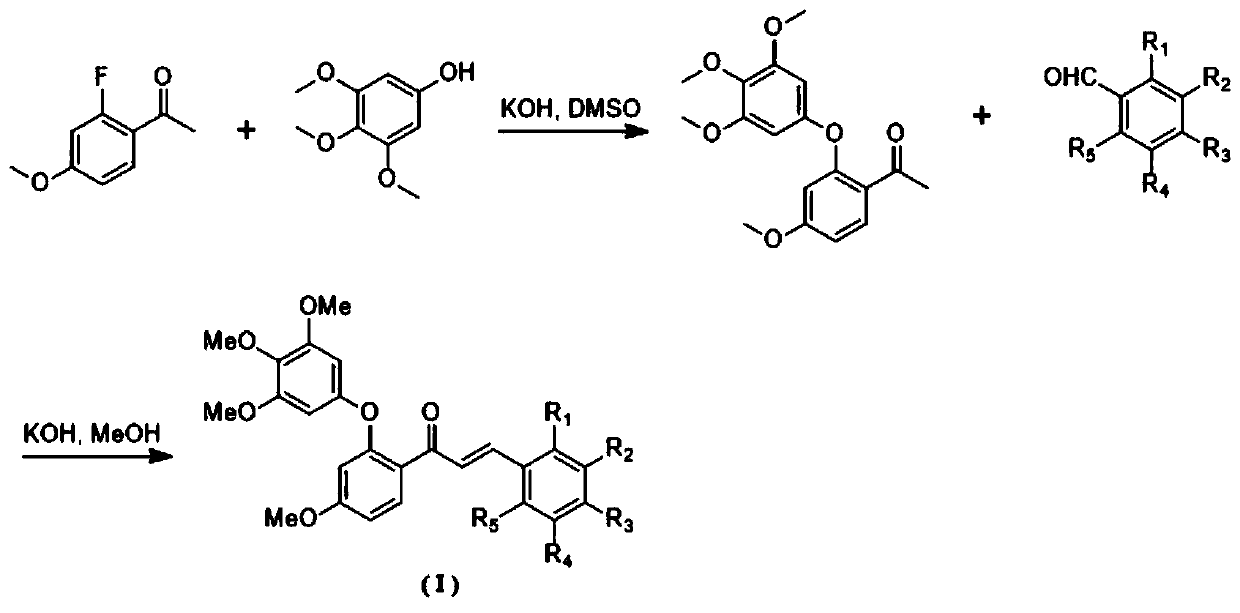
[0028] Step 1: 1-(2-fluoro-4-methoxyphenyl)ethan-1-one (1mmol), 3,4,5-trimethoxyphenol (1mmol), potassium hydroxide (2mmol), add 10ml of dimethyl sulfoxide was reacted at 100°C for 8 hours, cooled to room temperature, poured into water, extracted with ethyl acetate, spin-dried, separated and purified by silica gel column chromatography to obtain a solid powder.
[0029] Step 2: 1-(4-methoxy-2-(3,4,5-trimethoxyphenoxy)phenyl)ethan-1-one (1mmol), p-chlorobenzaldehyde (1mmol), methanol (10ml) was placed in a round-bottomed flask, added 3ml of 50% potassium hydroxide aqueous solution, stirred warmly for 48 hours, stopped the reaction, poured into water, adjusted the pH to neutral with dilute hydrochloric acid, filtered, dried, and recrystallized from ethanol to obtain a solid powder , yield 63%. 1 H NMR (CDCl 3 ,400MHz)δ:3.73(s,6H),3.77(s...
Embodiment 2
[0031] Example 2: (E)-3-(4-methoxyphenyl)-1-(4-methoxy-2-(3,4,5-trimethoxyphenoxy)phenyl)propane- Preparation of 2-en-1-one (2)
[0032]
[0033] Step 1: 1-(2-fluoro-4-methoxyphenyl)ethan-1-one (1mmol), 3,4,5-trimethoxyphenol (1mmol), potassium hydroxide (2mmol), add 10ml of dimethyl sulfoxide was reacted at 100°C for 8 hours, cooled to room temperature, poured into water, extracted with ethyl acetate, spin-dried, separated and purified by silica gel column chromatography to obtain a solid powder.
[0034] Step 2: 1-(4-methoxy-2-(3,4,5-trimethoxyphenoxy)phenyl)ethan-1-one (1mmol), p-methoxybenzaldehyde (1mmol) 1. Methanol (10ml) was placed in a round-bottomed flask, added 3ml of 50% potassium hydroxide aqueous solution, stirred warmly for 48 hours, stopped the reaction, poured into water, adjusted the pH to neutral with dilute hydrochloric acid, filtered, dried, and recrystallized from ethanol to obtain Solid powder, yield 52%. 1 H NMR (CDCl 3 ,400MHz)δ:3.73(s,6H),3.78(...
Embodiment 3
[0035] Example 3: (E)-3-(4-diethylaminophenyl)-1-(4-methoxy-2-(3,4,5-trimethoxyphenoxy)phenyl)propane Preparation of -2-en-1-one (3)
[0036]
[0037] Step 1: 1-(2-fluoro-4-methoxyphenyl)ethan-1-one (1mmol), 3,4,5-trimethoxyphenol (1mmol), potassium hydroxide (2mmol), add 10ml of dimethyl sulfoxide was reacted at 100°C for 8 hours, cooled to room temperature, poured into water, extracted with ethyl acetate, spin-dried, separated and purified by silica gel column chromatography to obtain a solid powder.
[0038] Step 2: Add 1-(4-methoxy-2-(3,4,5-trimethoxyphenoxy)phenyl)ethan-1-one (1mmol), p-diethylaminobenzaldehyde (1mmol ), methanol (10ml) was placed in a round-bottomed flask, 3ml of 50% aqueous potassium hydroxide solution was added, stirred warmly for 48 hours, the reaction was stopped, poured into water, adjusted to neutral pH with dilute hydrochloric acid, filtered, dried, ethanol recrystallized A solid powder was obtained with a yield of 46%. 1 H NMR (CDCl 3 ,400...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com