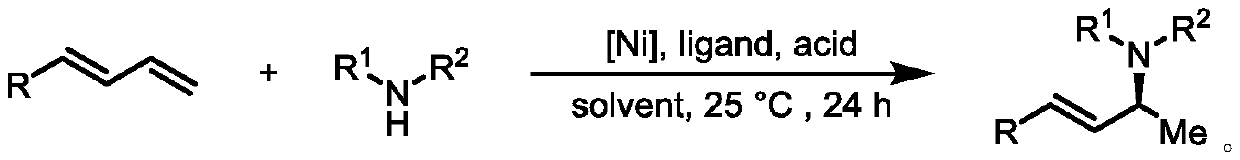
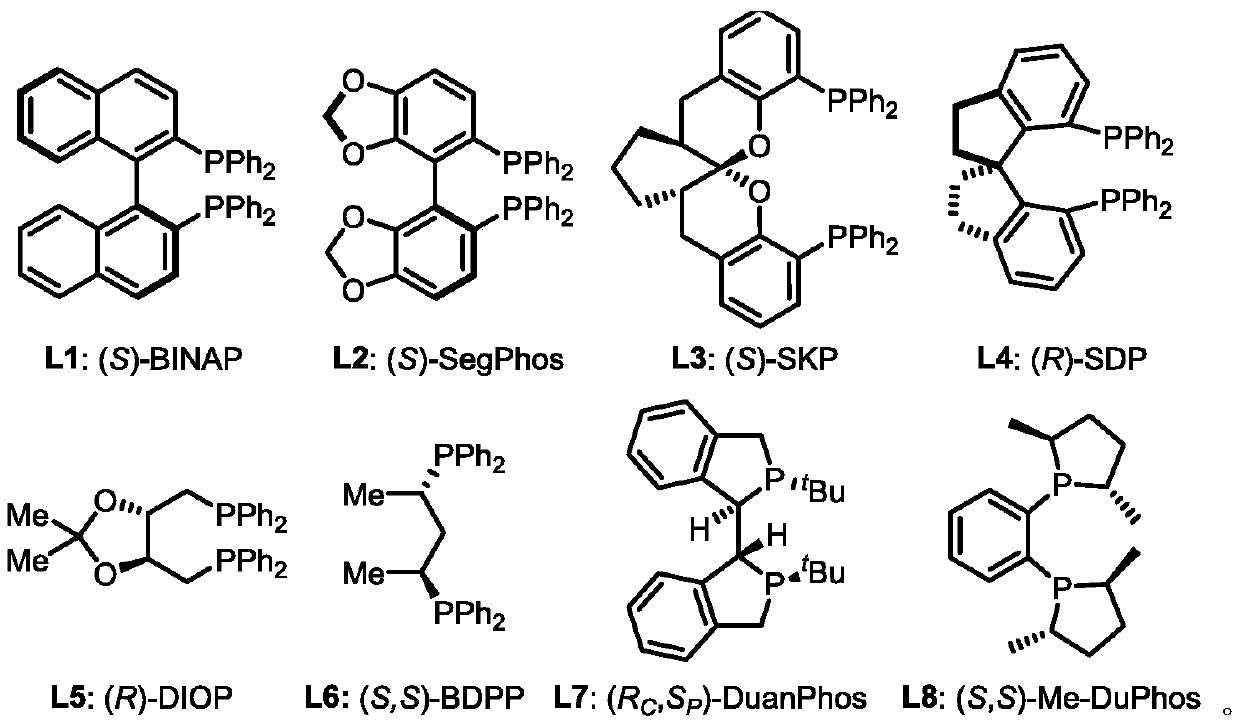
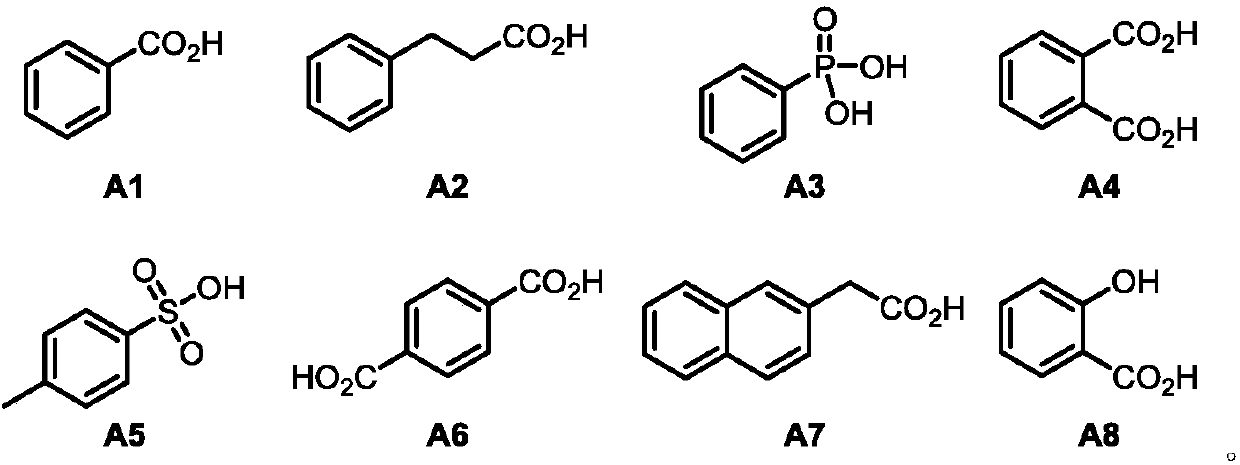
Method for preparing chiral allyl amine compound with high chemical and stereoselectivity
A technology of stereoselectivity and allylamine, which is applied in the direction of condensation/addition reaction to prepare amino compounds, organic chemical methods, chemical instruments and methods, etc., can solve the problems of narrow substrate range and poor functional group tolerance, and achieve operational Simple, efficient reaction, and mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031]
[0032] In a glove box filled with argon, bis(1,5-cyclooctadiene)nickel and (S,S)-Me-DuPhos were mixed in a molar ratio of 1:1 and dissolved in dry toluene to prepare A solution with a concentration of 0.01M (multiple reactions can be carried out at one time of complexation, and the catalyst solution is prepared according to the actual demand), pre-stirred for 0.5h. Then 1 mL of the catalyst solution was taken, and 1-phenyl-1,3-butadiene (26 μL, 0.2 mmol), n-butylamine (30 μL, 0.3 mmol) and phthalic acid (1.7 mg, 0.01 mmol) were added thereto , the reaction vial was sealed and taken out from the glove box, and reacted at 25 °C for 24 hours. After the reaction was completed, the reaction solvent was removed by concentration under reduced pressure, and the product (S,E)-N-butyl-4-phenylbut-3-en-2-amine was obtained by separation and purification by column chromatography, a colorless oily liquid with a yield of 93%; ee > 99%; [α]D 25 =-60.3 (c=1.0, CHCl 3 ); HPLC de...
Embodiment 2
[0034]
[0035] In a glove box filled with argon, bis(1,5-cyclooctadiene)nickel and (S,S)-Me-DuPhos were mixed in a molar ratio of 1:1 and dissolved in dry toluene to prepare A solution with a concentration of 0.01M (multiple reactions can be carried out at one time of complexation, and the catalyst solution is prepared according to the actual demand), pre-stirred for 0.5h. Then 1 mL of the catalyst solution was taken, and 1-phenyl-1,3-butadiene (26 μL, 0.2 mmol), 2-phenylethylamine (38 μL, 0.3 mmol) and phthalic acid (1.7 mg, 0.01 mmol), the reaction vial was sealed and taken out from the glove box, and reacted at 25°C for 24 hours. After the reaction was completed, the reaction solvent was removed by concentration under reduced pressure, and the product (S,E)-N-phenethyl-4-phenylbut-3-en-2-amine was obtained by separation and purification by column chromatography, which was a light yellow oily liquid with a yield of 99%; ee=92%; [α] D 25 =-76.8 (c=1.0, CHCl 3 ); HPLC ...
Embodiment 3
[0037]
[0038] In a glove box filled with argon, bis(1,5-cyclooctadiene)nickel and (S,S)-Me-DuPhos were mixed in a molar ratio of 1:1 and dissolved in dry toluene to prepare A solution with a concentration of 0.01M (multiple reactions can be carried out at one time of complexation, and the catalyst solution is prepared according to the actual demand), pre-stirred for 0.5h. Then 1 mL of the catalyst solution was taken, and 1-phenyl-1,3-butadiene (26 μL, 0.2 mmol), cyclopropylamine (21 μL, 0.3 mmol) and phthalic acid (1.7 mg, 0.01 mmol) were added thereto, The reaction vial was sealed and removed from the glove box, and reacted at 25°C for 24 hours. After the reaction was completed, the reaction solvent was removed by concentration under reduced pressure, and the product (S,E)-N-(4-phenylbut-3-en-2-yl)cyclopropanamine was obtained by separation and purification by column chromatography, a colorless oily liquid with a yield of 61% ;ee>99%;[α] D 25 =-91.8 (c=1.0, CHCl 3 );...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com