Application of cinnamate compounds in prevention and control of fungal diseases of crops
A technology of cinnamic acid esters and fungal diseases, which is applied in the field of pesticides, can solve the problems of crop injury and inhibit the normal growth of crops, and achieve the effect of avoiding injury and broad antibacterial spectrum
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
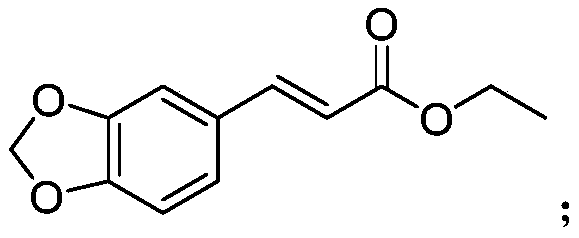
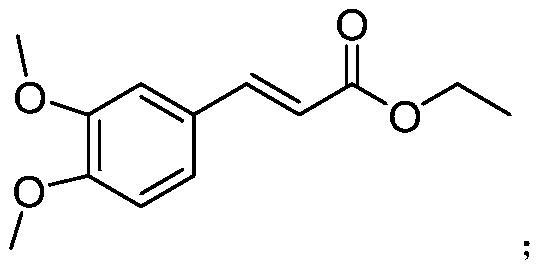
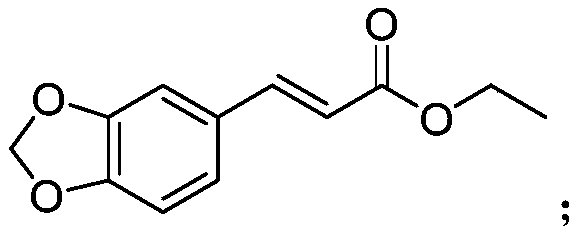
[0025] Example 1 3-(3'-fluoro-4'-methoxyphenyl) ethyl acrylate, 3-(3',4'-methylenedioxyphenyl) ethyl acrylate, 3-(3 Determination of antibacterial activity of ',4'-dimethoxyphenyl) ethyl acrylate and reference compound A in vitro
[0026] (1) Test drug
[0027] 3-(3'-fluoro-4'-methoxyphenyl) ethyl acrylate (purity 99%), 3-(3',4'-methylenedioxyphenyl) ethyl acrylate (purity 99%) %) and 3-(3',4'-dimethoxyphenyl) ethyl acrylate (purity 99%), control compound A (3,4-dimethoxycinnamic acid methyl ester, purity 99%), 96.5% chlorothalonil (non-systemic broad-spectrum fungicide), 97.2% carbendazim (systemic broad-spectrum fungicide), 97.0% procymidone (systemic broad-spectrum fungicide), 96.0 % Dimetholine ((E,Z)-4-[3-(4-chlorophenyl)3-(3,4-dimethoxyphenyl)acryloyl]morpholine, systemic fungicide) Original drug, 97.0% azoxystrobin original drug (systemic broad-spectrum fungicide).
[0028] (2) Plant pathogenic fungi tested
[0029] Rhizoctonia solani, Phytophthora infestans, Valsa...
Embodiment 2
[0057] Example 2 3-(3',4'-methylenedioxyphenyl)ethyl acrylate and 3-(3',4'-dimethoxyphenyl)ethyl acrylate with metalaxyl, Determination of Combined Toxicity Effects of Mixed Use of Meiling on Phytophthora capsici
[0058] (1) Test drug
[0059] 3-(3',4'-methylenedioxyphenyl)ethyl acrylate, 3-(3',4'-dimethoxyphenyl)ethyl acrylate, 98% metalaxyl technical, 99% Hymexazol original drug.
[0060] (2) Plant pathogenic fungi tested
[0061] Phytophthora capsici P. capsici.
[0062] (3) Toxicity determination method
[0063] 3-(3',4'-methylenedioxyphenyl)ethyl acrylate, 3-(3',4'-dimethoxyphenyl)ethyl acrylate (A) and metalaxyl, For the mixed use of Mycomethazol (B), five mass ratios of 3:1, 2:1, 1:1, 1:2, and 1:3 are set, and each ratio is formulated to correspond to 6 to 8 concentration gradients. The combined virulence assay of Phytophthora capsici was carried out by using the growth rate method. For the specific assay method, refer to Example 1. Measure the colony diameter a...
Embodiment 3
[0073] Example 3 3-(3'-fluoro-4'-methoxyphenyl) ethyl acrylate, 3-(3',4'-methylenedioxyphenyl) ethyl acrylate, 3-(3 ', 4'-Dimethoxyphenyl) ethyl acrylate and reference compound A control and control zucchini powdery mildew in vivo assay
[0074] (1) Preparation method of emulsifiable cream
[0075] Weigh 4.59g of ethyl 3-(3'-fluoro-4'-methoxyphenyl)acrylate, dissolve it in 25mL of xylene, add 6g of emulsifier 0203B, stir and mix to obtain 14% 3-(3 '-Fluoro-4'-methoxyphenyl) ethyl acrylate EC.
[0076] Weigh 4.59g of ethyl 3-(3',4'-methylenedioxyphenyl)acrylate, dissolve it in 25mL of xylene, add 6g of emulsifier 0203B, stir and mix to obtain 14% 3-( 3',4'-Methylenedioxyphenyl) ethyl acrylate EC.
[0077] Weigh 3.16g of ethyl 3-(3',4'-dimethoxyphenyl)acrylate, dissolve it in 25mL of xylene, add 6g of emulsifier 0203B, stir and mix to obtain 10% 3-(3' , 4'-dimethoxyphenyl) ethyl acrylate EC.
[0078] Weigh 4.59g of reference compound A, dissolve it in 25mL of xylene, add 6g...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com