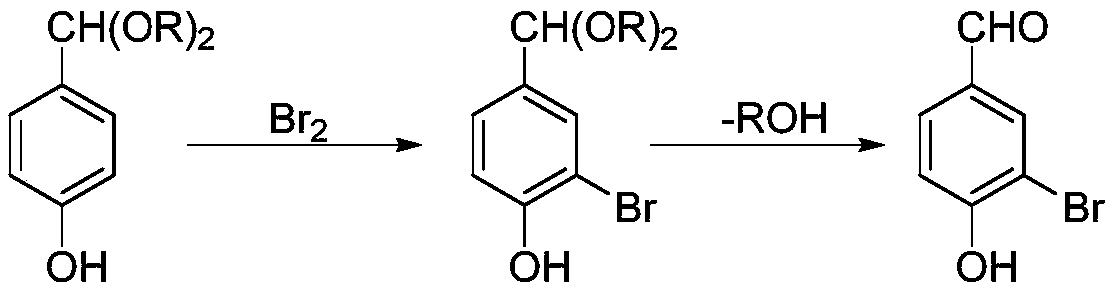
Preparation method of 3-bromo-4-hydroxybenzaldehyde
A technology of hydroxybenzaldehyde and hydroxybenzyl acetal, which is applied in the field of preparation of 3-bromo-4-hydroxybenzaldehyde, can solve the problems of difficult separation, high recovery energy consumption, and low yield, and achieve a simple process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) Acetal reaction
[0038] Dissolve 61g of p-hydroxybenzaldehyde in 600g of ethanol, react at 25°C for 4h, then use an 80cm glass spring rectification column, R=3, heat the azeotropic band water (adjust the pressure so that the temperature of the kettle is lower than 30°C), to Stop when the water content of the still liquid is lower than 0.5%, and obtain 219 g of an alcoholic solution of p-hydroxybenzyl acetal.
[0039] (2) bromination reaction
[0040] Weigh 80g of bromine solution and dissolve it in 200g of ethanol, mix well, add dropwise to the alcohol solution obtained in step (1), the dropwise addition time is 2h, and the reaction temperature is -20°C, continue the reaction for 4h after the dropwise addition is completed, 500 g of the bromination reaction solution was obtained.
[0041] (3) Hydrolysis reaction
[0042] Add 600g of water to the bromination reaction solution, heat to reflux for 4h (80°C), cool down to crystallize (-20°C), filter to obtain 98.5g ...
Embodiment 2
[0044] (1) Acetal reaction
[0045] Dissolve 61g of p-hydroxybenzaldehyde in 120g of tert-butanol solution, stir, fully react for 3h at 20°C, then use a 40cm glass spring tower, R=2, azeotropic water (properly adjust the pressure so that the temperature of the kettle is not higher than 25 ℃) until the water content of the kettle liquid is lower than 0.5%, to obtain 110 g of a mixture of acetal and tert-butanol.
[0046] (2) bromination reaction
[0047] Weigh 84g of bromine solution and dissolve it in 200g of tert-butanol, mix well, add dropwise to the acetalized tert-butanol solution, the time for adding is about 2 hours, and the reaction temperature is -10°C, continue the reaction after the addition is completed After 3h, 394g of bromination reaction solution was obtained.
[0048] (3) Hydrolysis reaction
[0049] Add 600g of water to the bromination reaction solution, heat to reflux for 4h (85°C), cool down and crystallize (10°C), filter to obtain 99.4g of 3-bromo-4-hydr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com