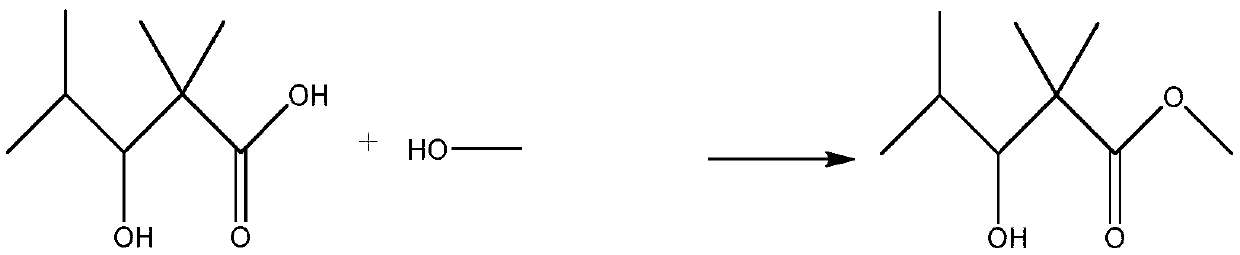Preparation method of 2,2,4-trimethyl-3-hydroxypentanoate, waterborne industrial paint for steel structure and preparation method of waterborne industrial paint
A technology of methyl hydroxyvalerate and hydroxyvaleric acid, which is used in the synthesis and application of organic compounds, can solve the problems of strong taste, low VOC content, and high VOC content of water-based industrial paint for steel structures, and achieve low production costs and simplified production The effect of high process and reaction rate
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0031] A preparation method of 2,2,4-trimethyl-3 hydroxyvaleric acid methyl ester, comprising the following steps:
[0032] 1) Preparation of 2,2,4-trimethyl-3-hydroxypentanoic acid
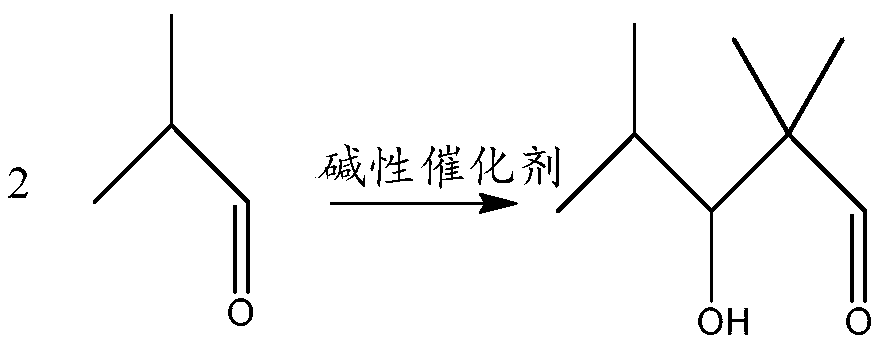
[0033] Isobutyraldehyde undergoes an aldol condensation reaction under the action of a basic catalyst to generate 2,2,4-trimethyl-3-hydroxypentanal. The reaction formula is as follows:
[0034] 2
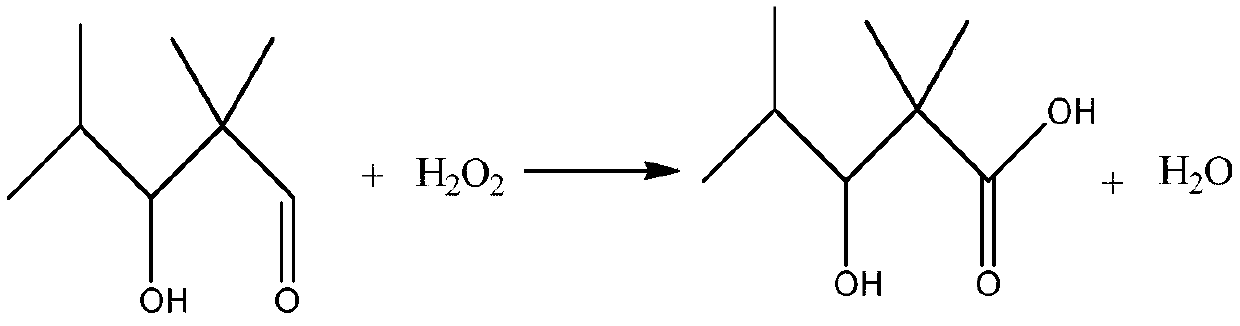
[0035] The 2,2,4-trimethyl-3-hydroxypentanal obtained above was mixed with H 2 o 2Oxidation reaction occurs to obtain 2,2,4-trimethyl-3 hydroxypentanoic acid, the reaction formula is as follows:
[0036]
[0037] 2) Preparation of 2,2,4-trimethyl-3-hydroxypentanoic acid methyl ester
[0038] The 2,2,4-trimethyl-3 hydroxyvaleric acid and methanol obtained in step 1) are reacted under the action of a strong acid catalyst to obtain 2,2,4-trimethyl-3 hydroxyvaleric acid methyl ester, and the reaction formula is as follows :
[0039]
[0040] In the step 1), the basic catalyst is NaOH, KOH, Ca...
Embodiment 1
[0048] The preparation method of the 2,2,4-trimethyl-3 hydroxyvaleric acid methyl ester of the present embodiment comprises the following steps:
[0049] 1) Preparation of 2,2,4-trimethyl-3-hydroxypentanoic acid
[0050] With 600g isobutyraldehyde and 45 grams, mass fraction is 40% Ba(OH) 2 Add the solutions into the reaction kettle respectively, stir and raise the temperature to 55°C. After reacting for 7 hours, take samples for gas chromatography test at intervals of 30 minutes. When 2,2,4-trimethyl-3-hydroxypentanal Stop the reaction when the content of isobutyraldehyde no longer increases, then distill off the unreacted isobutyraldehyde at 70°C and -0.1MPa, then wash with water, separate the oil phase product, and obtain 520g of 2,2,4-trimethyl- 3-Hydroxypentanal.
[0051] Add the 2,2,4-trimethyl-3-hydroxyvaleraldehyde obtained above into the reaction kettle, raise the temperature to 80°C, and add hydrogen peroxide dropwise while reacting. 2 o 2 The mass fraction of th...
Embodiment 2
[0057] The preparation method of the 2,2,4-trimethyl-3 hydroxyvaleric acid methyl ester of the present embodiment comprises the following steps:
[0058] 1) Preparation of 2,2,4-trimethyl-3-hydroxypentanoic acid
[0059] Add 700g of isobutyraldehyde and 60g of KOH solution with a mass fraction of 35% into the reaction kettle respectively, stir and raise the temperature to 65°C, after reacting for 2 hours, take samples at 30-minute intervals for gas chromatography test, when two consecutive chromatographic tests Stop the reaction when the content of 2,2,4-trimethyl-3-hydroxypentanal no longer increases, then distill off the unreacted isobutyraldehyde at 65°C and -0.05MPa, then wash with water, and separate the oil phase product, 620g of 2,2,4-trimethyl-3-hydroxypentanal was obtained.
[0060] Add the 2,2,4-trimethyl-3-hydroxyvaleraldehyde obtained above into the reaction kettle, raise the temperature to 50°C, and add hydrogen peroxide dropwise while reacting. 2 o 2 The mass ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| boiling point | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
| quality score | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com