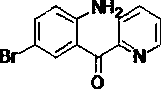
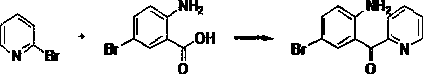Synthesis method of 2-(2-amino-5-bromobenzoyl) pyridine
A technology of a benzoyl group and a synthesis method, which is applied in the synthesis field of 2-pyridine, can solve problems such as inconvenience and production danger, and achieve the effects of high yield and purity, little environmental pollution, and simple and easy handling.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
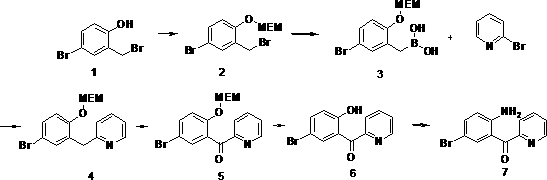
[0037] S1. Synthesis of compound 4-bromo-2-(bromomethyl)-1-((2-methoxyethoxy)methoxy)benzene
[0038] In a 3L three-necked flask, add 263.8g (1.0mol) of 4-bromo-2-bromomethylphenol and 202.4g (2.0mol) of acid-binding agent triethylamine into 1L of dichloromethane, keep at 25°C, and add dropwise MEMCl 149.5 g (1.2 mol). Keep stirring at 25° C. for 6 h after dropping, and HPLC detects that the reaction is complete. The reaction solution was filtered to remove salt, the organic phase was washed several times with water, dried over anhydrous sodium sulfate, and concentrated to dryness to obtain 352 g, with a yield of 99.4%.
[0039] S2. Synthesis of compound (5-bromo-2-(((2-methoxyethoxy)methoxy)benzyl)boronic acid
[0040] In a 3L three-necked flask, sequentially feed THF1L, Mg6.86g (0.28mol), triethylamine 85.6g (0.84mol), trimethyl borate 34.9g (0.34mol), after adding, keep room temperature, dropwise add 4- Bromo-2-(bromomethyl)-1-((2-methoxyethoxy)methoxy)benzene 100g (0.28...
Embodiment 2
[0050] Example 2: Synthesis of S1, compound 4-bromo-2-(bromomethyl)-1-((2-methoxyethoxy)methoxy)benzene
[0051] In a 3L three-necked flask, add 263.8g (1.0mol) of 4-bromo-2-bromomethylphenol and 138.2g (1.0mol) of potassium carbonate as an acid-binding agent into 1L of acetonitrile, keep at 25°C, and add 149.5g of MEMCl dropwise (1.2 mol). Keep stirring at 25° C. for 6 h after dropping, and HPLC detects that the reaction is complete. The reaction solution was filtered to remove salt, the organic phase was washed several times with water, dried over anhydrous sodium sulfate, and concentrated to dryness to obtain 350 g, with a yield of 98.8%.
[0052] S2. Synthesis of compound (5-bromo-2-(((2-methoxyethoxy)methoxy)benzyl)boronic acid
[0053] In a 3L three-necked flask, sequentially feed THF1L, Mg6.86g (0.28mol), triethylamine 85.6g (0.84mol), trimethyl borate 34.9g (0.34mol), after adding, keep room temperature, dropwise add 4- Bromo-2-(bromomethyl)-1-((2-methoxyethoxy)meth...
Embodiment 3
[0063] Example 3: Synthesis of S1, compound 4-bromo-2-(bromomethyl)-1-((2-methoxyethoxy)methoxy)benzene
[0064] In a 3L three-necked flask, 263.8g (1.0mol) of 4-bromo-2-bromomethylphenol and 202.4g (2.0mol) of acid-binding agent triethylamine were added to 1L of dimethylformamide and kept at 25°C. 149.5 g (1.2 mol) of MEMCl was added dropwise. Keep stirring at 25° C. for 6 h after dropping, and HPLC detects that the reaction is complete. The reaction solution was filtered to remove salt, the organic phase was washed several times with water, dried over anhydrous sodium sulfate, and concentrated to dryness to obtain 349 g, with a yield of 98.5%.
[0065] S2. Synthesis of compound (5-bromo-2-(((2-methoxyethoxy)methoxy)benzyl)boronic acid
[0066] In a 3L three-necked flask, sequentially feed THF1L, Mg6.86g (0.28mol), triethylamine 85.6g (0.84mol), trimethyl borate 34.9g (0.34mol), after adding, keep room temperature, dropwise add 4- Bromo-2-(bromomethyl)-1-((2-methoxyethoxy)...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com