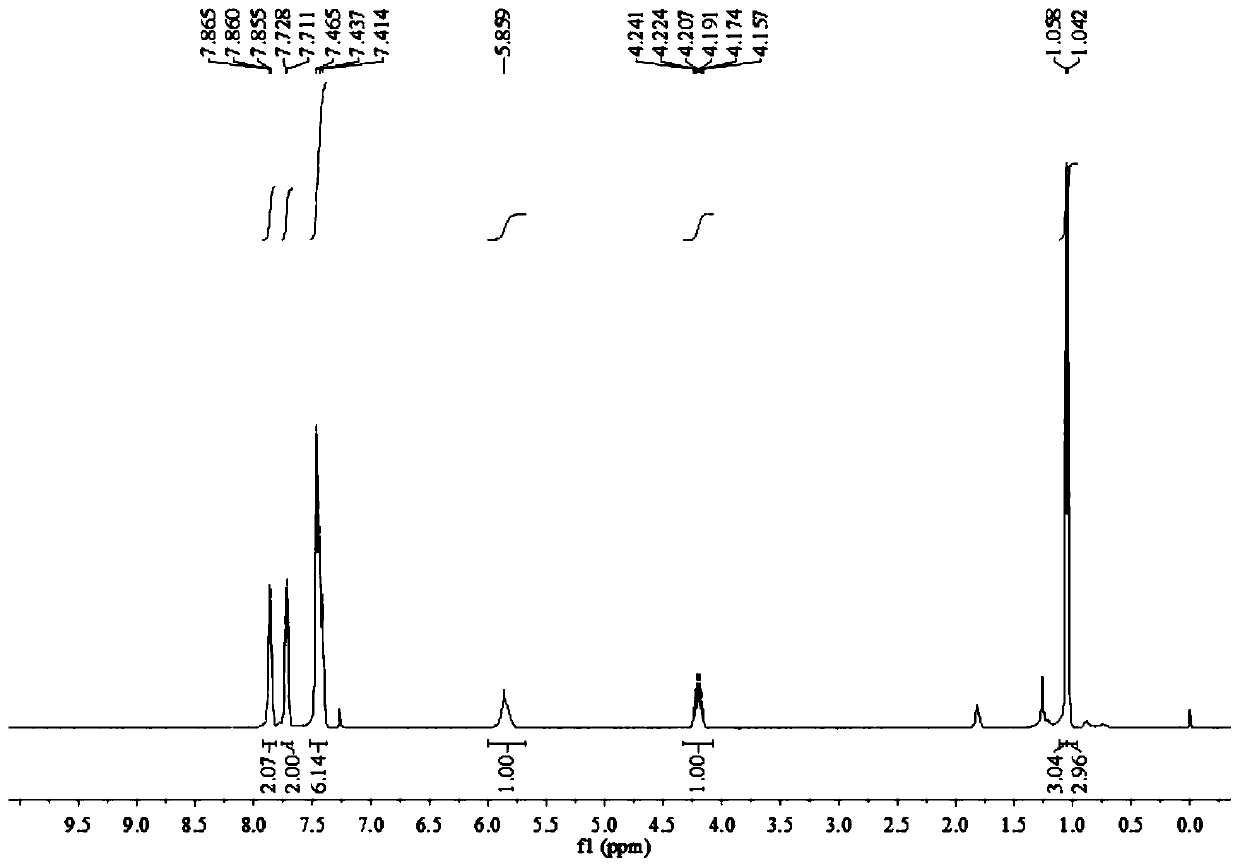
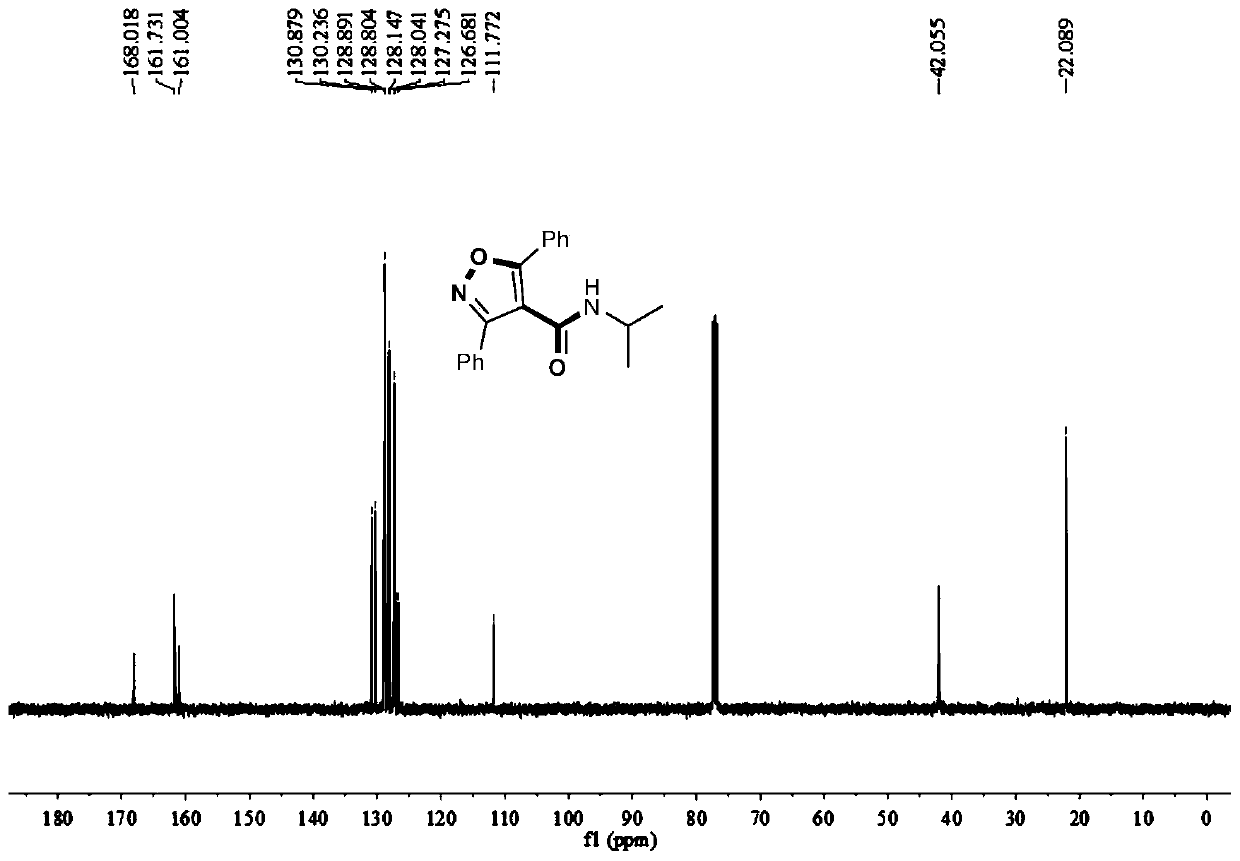
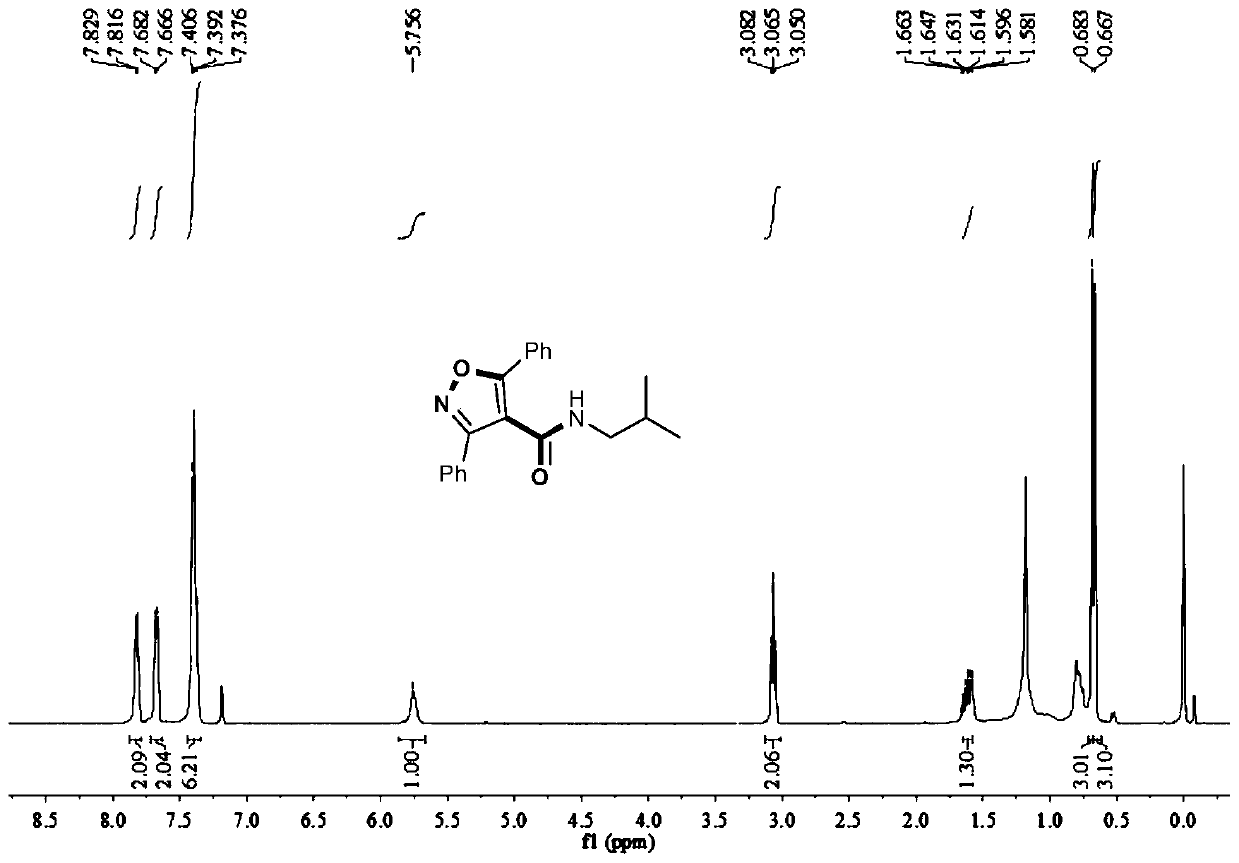
Method for synthesizing 4-amide isooxazole
A technology for isoxazole and amide, which is applied in the field of synthesizing 4-amidoisoxazole, can solve the problems that 4-amidoisoxazole derivatives have not been directly synthesized, and achieve a wide range of substrate universality, The method is simple and easy, and the effect of mild reaction conditions
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] In a 15mL round bottom flask, add 0.003mol bis(acetonitrile) dichloropalladium, 2 equivalents of sodium iodide, 0.10mmol 1,3-diphenyl O-methyl acetylenone oxime ether, 0.40mmol isopropylamine, 1mL DMSO, filled with carbon monoxide balloon, stirred and reacted at 40°C for 12 hours, stopped heating and stirring, cooled to room temperature, and distilled under reduced pressure to obtain the crude product, and then separated and purified by column chromatography to obtain the target product, which was eluted by column chromatography The liquid is petroleum ether with a volume ratio of 20:1: ethyl acetate mixed solvent, and the yield is 15%.
Embodiment 2
[0034] In a 15mL two-necked round bottom flask, add 0.003mol allyl palladium chloride dimer, 2 equivalents of sodium iodide, 0.10mmol 1,3-diphenyl O-methyl acetylene ketoxime ether, 0.40mmol isopropyl Amine, 1mL DMSO, filled with a carbon monoxide balloon, stirred at 40°C for 12 hours, stopped heating and stirring, cooled to room temperature, and distilled under reduced pressure to obtain a crude product, which was then separated and purified by column chromatography to obtain the target product. The eluent was analyzed as petroleum ether:ethyl acetate mixed solvent with a volume ratio of 20:1, and the yield was 11%.
Embodiment 3
[0036] Add 0.003mol of bis(pyridine)palladium dichloride, 2 equivalents of sodium iodide, 0.10mmol of 1,3-diphenyl O-methyl acetylene ketone oxime ether, 0.40mmol of isopropylamine in a 15mL two-neck round bottom flask, 1mL DMSO, filled with carbon monoxide balloon, stirred and reacted at 40°C for 12 hours, stopped heating and stirring, cooled to room temperature, and distilled under reduced pressure to obtain the crude product, then separated and purified by column chromatography to obtain the target product, the column chromatography used The deliquification was petroleum ether: ethyl acetate mixed solvent with a volume ratio of 20:1, and the yield was 30%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com