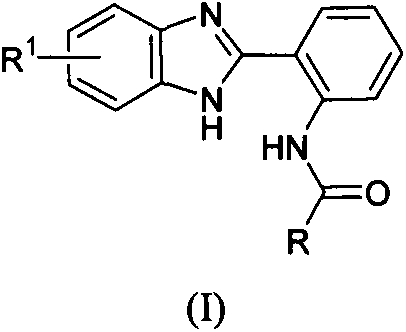
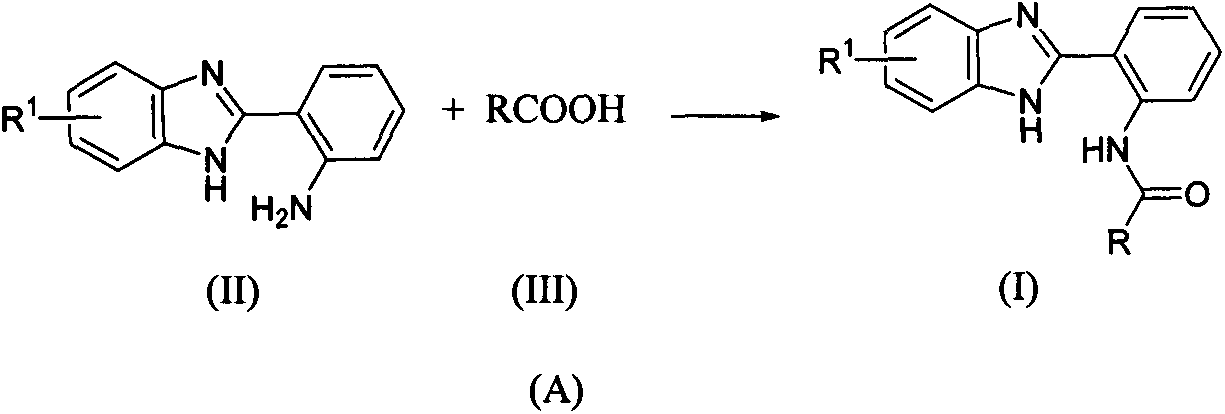
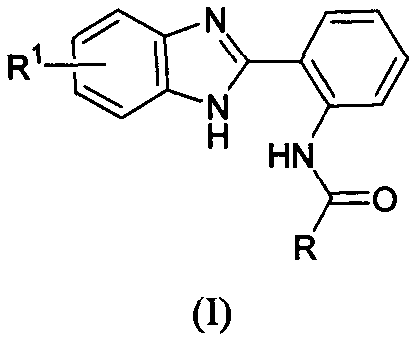
Benzimidazolyl containing amide derivative, preparation method therefor and application of benzimidazolyl containing amide derivative
A technology based on benzimidazole and benzimidazole, which is applied in the field of prevention and treatment of plant fungal diseases, can solve the problems of limiting the use and development of fungicides, single targets, and long-term damage, and achieves the goal of inhibiting and preventing agricultural fungal diseases and responding Conditions are easy to control and raw materials are easy to obtain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] Example 1: N-(2-(1H-benzimidazol-2-yl)phenyl)-5-chloro-1,3-dimethyl-1H-pyrazole-4-carboxamide Preparation of (I1)
[0039] 5-Chloro-1,3-dimethyl-1H-pyrazole-4-carboxylic acid (0.52g, 3mmol) was slowly added into 10mL of thionyl chloride, refluxed for 6h, evaporated under reduced pressure to remove thionyl chloride, added 20mL of di Chloromethane, cooled to -5°C, slowly added 2-(1H-benzimidazol-2-yl)aniline (0.63g, 3mmol) in batches, added triethylamine (0.60g, 6mmol), stirred for 1h, naturally Warm up to room temperature and continue stirring for 4h. The reaction solution was washed with water, washed with saturated brine, washed with water, dried, evaporated to remove the solvent under pressure, and purified by column chromatography to obtain N-(2-(1H-benzimidazol-2-yl)phenyl)-5-chloro -1,3-Dimethyl-1H-pyrazole-4-carboxamide (I1).
Embodiment 2
[0040] Example 2: N-(2-(6-chloro-1H-benzimidazol-2-yl)phenyl)-5-chloro-1-methyl-3-trifluoromethyl-1H- Preparation of pyrazole-4-carboxamide (I8)
[0041] 5-Chloro-1-methyl-3-trifluoromethyl-1H-pyrazole-4-carboxylic acid (0.68g, 3mmol) was slowly added to 10mL of thionyl chloride, refluxed for 6h, and the thionyl chloride was distilled off under reduced pressure , add 20mL of dichloromethane, cool to -5°C, slowly add 2-(6-chloro-1H-benzimidazol-2-yl)aniline (0.73g, 3mmol) in batches, add triethylamine (0.60g, 6mmol), stirred for 1h, naturally warmed to room temperature, and continued to stir for 6h. The reaction solution was washed with water, washed with saturated brine, washed with water, dried, evaporated to remove the solvent under pressure, and purified by column chromatography to obtain N-(2-(5-chloro-1H-benzimidazol-2-yl)phenyl) -5-Chloro-1-methyl-3-trifluoromethyl-1H-pyrazole-4-carboxamide (I8).
Embodiment 3
[0042] Example 3: Preparation of N-(2-(6-chloro-1H-benzimidazol-2-yl)phenylnicotinic acid amide (I11)
[0043] Nicotinic acid (0.37g, 3mmol) and TBTU (1.15g, 3.6mmol) were dissolved in dichloromethane, triethylamine (0.60g, 6mmol) was added, stirred for 30min, and 2-(6-chloro-1H-benzo Imidazol-2-yl)aniline (0.63g, 3mmol), continue to stir for 10h, a large amount of solids are produced in the reaction mixture, filter with suction, wash with water, and dry to obtain N-(2-(6-chloro-1H-benzimidazole- 2-yl)phenylnicotinic acid amide (I11).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com