Method for preparing bremelanotide by solid-liquid combination
A solid-phase peptide synthesis, solid-state technology, applied in peptide preparation methods, chemical instruments and methods, organic chemistry, etc., can solve the problems of unfavorable large-scale production, expensive raw materials, high cost of large-scale production, etc., and achieve improved purification yield High efficiency and product final purity, high crude product purity and yield, and good economic and social value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
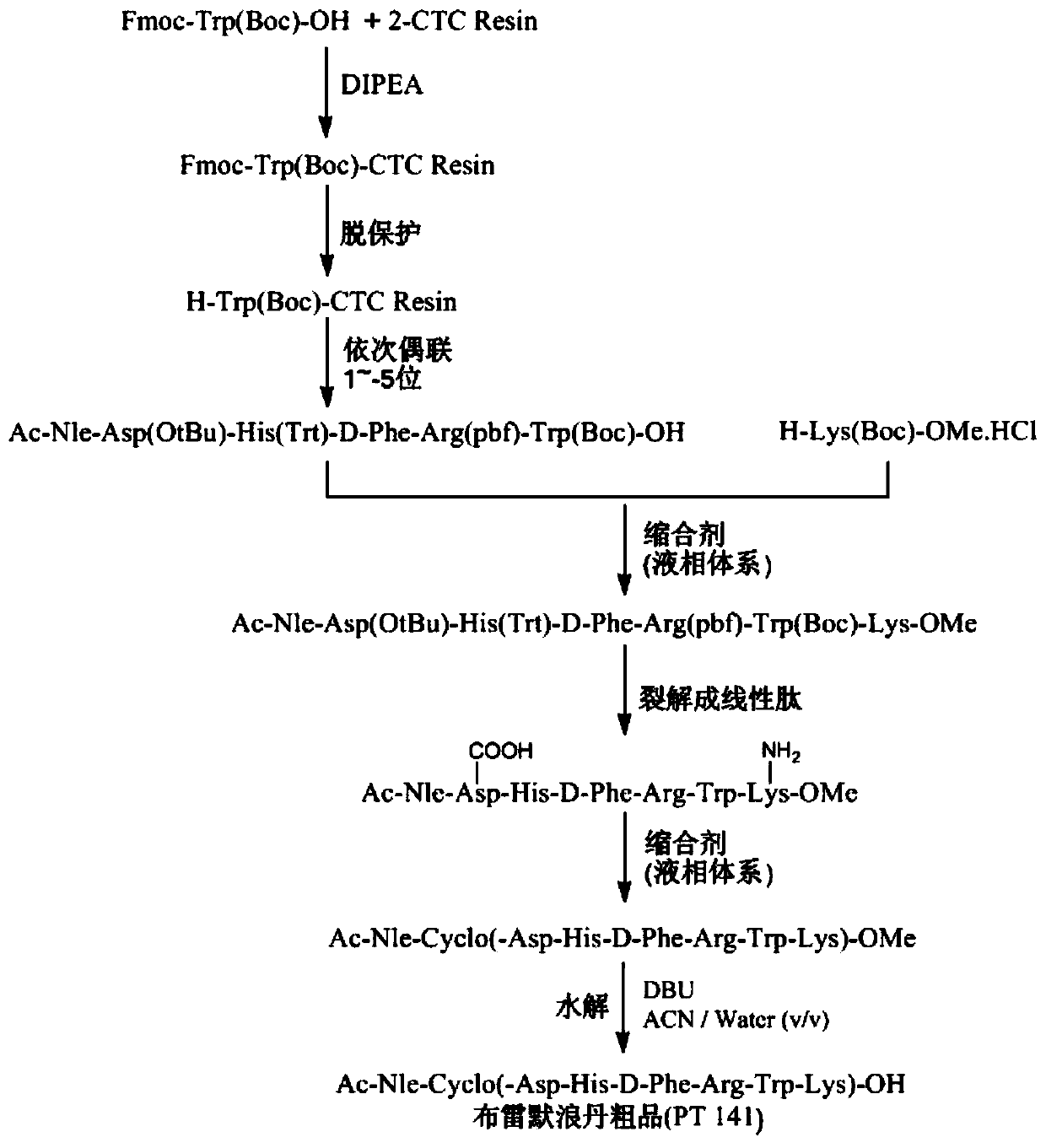
[0041] 1. Synthesis of fully protected hexapeptide Ac-Nle-Asp(OtBu)-His(Trt)-D-Phe-Arg(pbf)-Trp(Boc)-OH
[0042] Weigh 30.0g 2-CTC Resin (1.04mmol / g) in a solid-phase peptide synthesis reactor, add DCM to dissolve it for 30min, then pump it off, weigh 19.716g Fmoc-Trp(Boc)-OH and add about 120mL DMF to dissolve it while ice Cool down in a water bath, add 5.434 mL DIPEA to activate for 10 minutes, then add the amino acid activation solution to the reaction column for reaction, then add 3.804 mL DIPEA to the reactor to continue the reaction for about 1 hour. After the reaction, add DMF, MTBE, and DMF alternately to wash twice , pour the pre-prepared blocking solution DCM / MeOH / DIPEA=17 / 2 / 1 (V / V / V) into the resin to block the unreacted functional groups, block twice, each time for 10min, after the block is completed, Add DMF to wash, shrink the resin with MTBE, transfer to a vacuum drying oven to dry to constant weight, take the resin and measure the degree of substitution to be 0...
Embodiment 2
[0053]1. Synthesis of fully protected hexapeptide Ac-Nle-Asp(OtBu)-His(Trt)-D-Phe-Arg(pbf)-Trp(Boc)-OH
[0054] Weigh 30.0g 2-CTC Resin (1.04mmol / g) in a solid-phase peptide synthesis reactor, add DCM to swell for 30min, pump it off, weigh 24.64g Fmoc-Trp(Boc)-OH and add about 120mL DMF to dissolve it on ice Cool down in a water bath, add 9.238mL DIPEA to activate for 10 minutes, then add the amino acid activation solution to the reaction column for reaction, then add 7.065mL DIPEA to the reactor to continue the reaction for about 1 hour. After the reaction, add DMF, MTBE, and DMF alternately to wash twice , pour the pre-prepared blocking solution DCM / MeOH / DIPEA=17 / 2 / 1 (V / V / V) into the resin to block the unreacted functional groups, block twice, each time for 10min, after the block is completed, Add DMF to wash, shrink the resin with MTBE, transfer to a vacuum drying oven to dry to constant weight, take the resin and measure the degree of substitution to be 0.76 mmol / g.
[00...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com