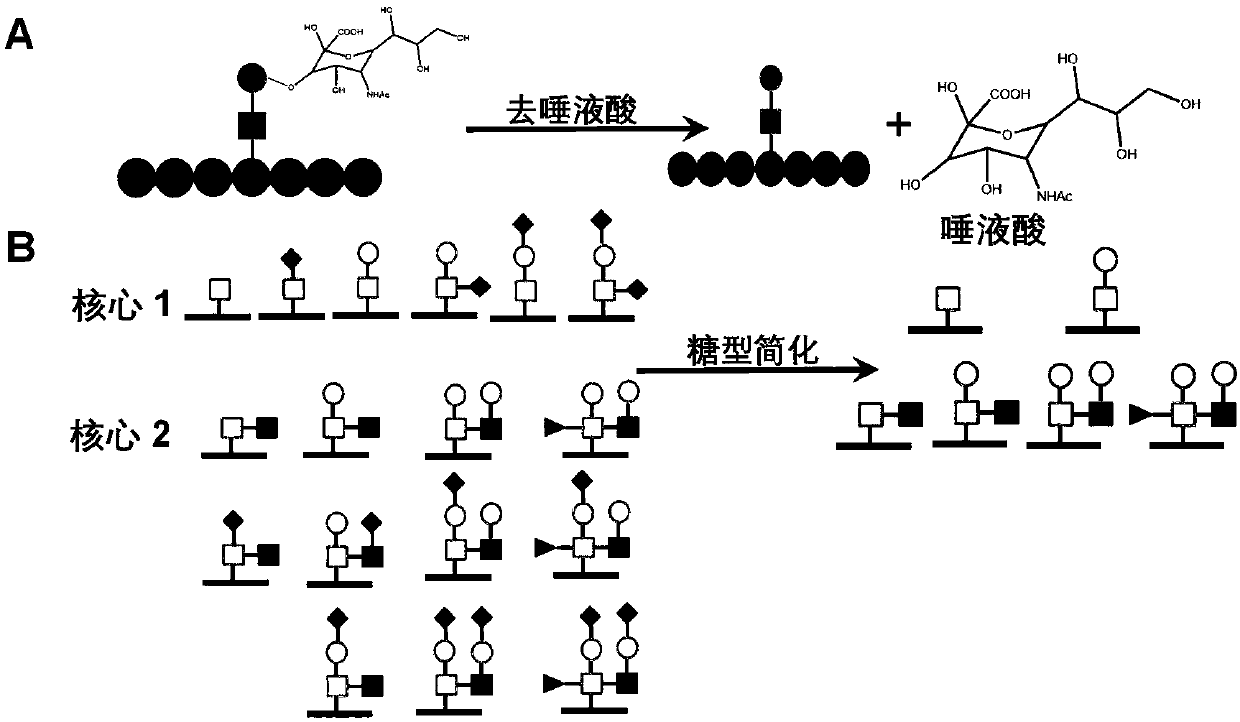
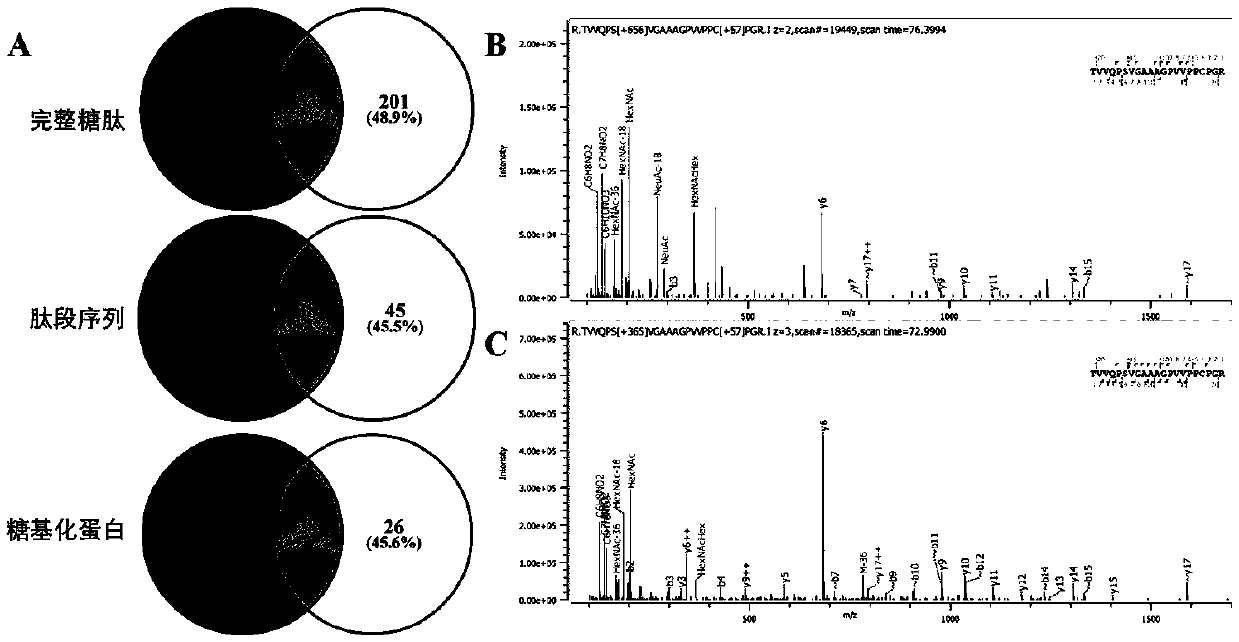
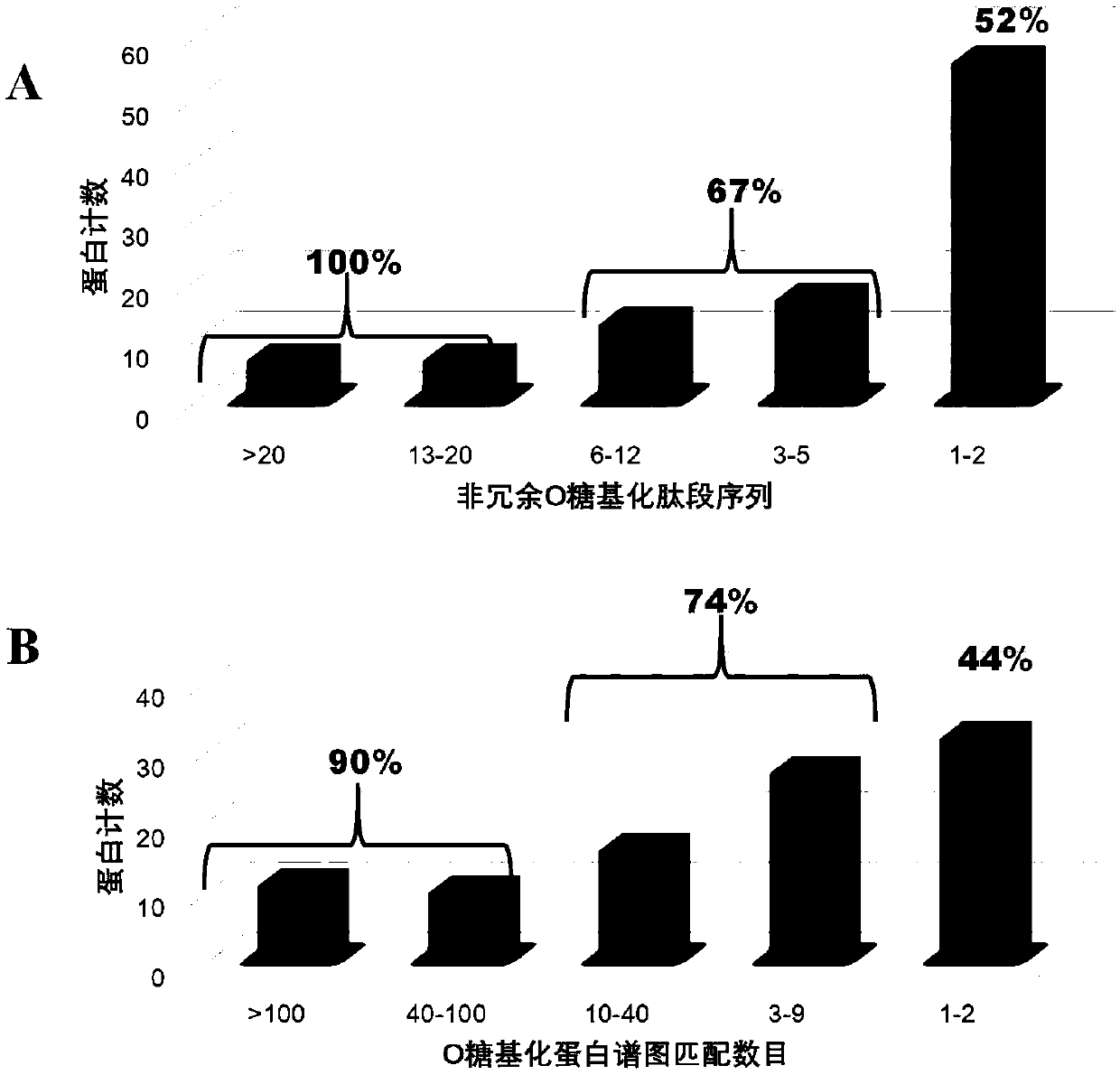
Method for identifying simplified O-GalNAc glycosylation peptide fragment based on chemical reaction
A technology of chemical reaction and identification method, which is applied in the field of mass spectrometry acquisition in the field of proteomics research, can solve the problems of not reflecting the real state of cells, complex, unsuitable tissue and body fluid samples, etc., and achieves low price, short reaction time and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Low-abundance peptide analysis method in micro-system for proteomics analysis of human serum:
[0025] (1) Dissolve human serum in a buffer solution containing 8M Urea and 50mM HEPES, add a final concentration of 20mM MDTT, react in a water bath at 37°C for 2h, then add IAA with a final concentration of 40mM, and react in the dark at 25°C for 40min;
[0026] (2) For the sample obtained after step (1), dilute the concentration of Urea to 1 M with an aqueous solution of 50 mM HEPES, then add Trypsin with a mass ratio of 1 / 20 to the protein, and enzymolyze it in a water bath at 37°C for 20 h, Obtain peptide solution;
[0027] (3) Adjust the pH value of the sample obtained after step (2) to about 2 with TFA, and select the corresponding C 18 The solid-phase extraction column removes small molecules in the solution, and the peptide eluate is freeze-dried;
[0028] (4) Redissolve the peptide obtained in step (3) in 50 mM sodium phosphate solution (pH=7.5), add PNGase F with...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com